Trichoptera
Caddisflies
Ralph W. Holzenthal, Roger J. Blahnik, Aysha Prather, and Karl Kjer

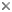
This tree diagram shows the relationships between several groups of organisms.
The root of the current tree connects the organisms featured in this tree to their containing group and the rest of the Tree of Life. The basal branching point in the tree represents the ancestor of the other groups in the tree. This ancestor diversified over time into several descendent subgroups, which are represented as internal nodes and terminal taxa to the right.

You can click on the root to travel down the Tree of Life all the way to the root of all Life, and you can click on the names of descendent subgroups to travel up the Tree of Life all the way to individual species.
For more information on ToL tree formatting, please see Interpreting the Tree or Classification. To learn more about phylogenetic trees, please visit our Phylogenetic Biology pages.
close boxIntroduction
Trichoptera, or caddisflies, comprise the most diverse insect order whose members are exclusively aquatic. Only aquatic Diptera outnumber them in species and ecological diversity. The larval stages are found in lakes, rivers, and streams around the world, and are important components of food webs in these freshwater ecosystems (Resh and Rosenberg 1984). A few species in the family Chathamiidae from New Zealand and Australia are unusual for the Insecta in having larvae that are truly marine, mostly restricted to tidal pools.
Adult Trichoptera, in contrast to the larvae, are terrestrial and look much like drab, fragile moths, often occurring in large numbers in lakeside or streamside habitats. The similarity is not incidental. Trichoptera are closely related to the order Lepidoptera and together the two orders comprise the superorder Amphiesmenoptera, or "dressed-up wings," the name referring to the dense clothing of scales or hairs on the wings. Monophyly of these two orders is strongly supported in both morphological and molecular analyses (Kristensen 1984, Wheeler, et al. 2001). Trichoptera possess the more primitive character state, having hairs rather than scales, and this character accounts for the name Trichoptera, meaning "hairy wings." Also, unlike moths and butterflies, which typically have a coiled, tubelike proboscis for feeding, adult caddisflies lack well-developed mouthparts, including the absence of mandibles, but have a well-developed haustellum (synapomorphic for the order) formed from a fusion of the hypopharynx and labium, and used in some species to imbibe liquids.
Unlike Lepidoptera larvae, which are predominantly terrestrial herbivores, Trichoptera larvae, with very few exceptions, are aquatic and primarily detritivorous. Like lepidopteran caterpillars, caddisfly larvae are capable of spinning silk from specially modified salivary glands. The diversity of microhabitats exploited by caddisfly larvae is a consequence of the many ways silk is used to construct retreats, nets, and cases and probably accounts for the success of the order as a whole (Mackay and Wiggins 1979, Wiggins 1996).
Almost 12,000 caddisfly species, placed in 45 families and about 600 genera, have been described from all faunal regions, but it has been estimated that the world fauna may contain as many as 50,000 species (Schmid 1984). The three currently recognized suborders are largely characterized by differences in the way silk is used (Ross 1944), whether to produce nets or tubes, or as glue to make various types of portable cases, often incorporating sand and small pebbles, or bits of leaves and twigs, each genus or even species building its own particular style of case. Some larvae are free-living and predaceous, but nevertheless lay down a strand of silk as they move, much like the larvae of Lepidoptera.
The larvae, and the fascinating nets and cases they produce, represents the life stage most familiar to the non-entomologist, and the case-making behavior of some species may account for the English common name, caddisfly. Although the origin of the word is obscure, it has been suggested to derive from cadaz or cadace (caddys), a word of variable spelling used in Shakespearean times to refer to a ribbon made from a certain kind of yarn sold by traveling vendors, who because of this were sometimes called "cadice men." Cadice men would pin samples of their wares to their clothing, a habit which may have suggested the name caddisfly or caddisworm for the aquatic larvae, who exhibit the analogous behavior of attaching bits of leaves and twigs to the outside of their cases (Hickin 1967).
Although caddisflies are not generally considered to be of great economic importance as pests, they are beneficially important in the trophic dynamics and energy flow in aquatic ecosystems. The larvae are also useful as biological indicator organisms for assessing water quality. Extensive use of them has been made for this purpose because larvae of different species vary in sensitivity to various types of pollution (Resh and Unzicker 1975, Resh 1993, Dohet 2002), and because the taxonomy of the group is relatively well known for temperate regions. Unfortunately, the larvae of many species, especially in the tropics, are unknown or have not been associated with their adult forms.
Characteristics
Caddisflies are easily recognized by a number of features. Adult mouthparts are reduced, with mandibles essentially absent, but the maxillary and labial palps, and often the haustellum, are prominent. The compound eyes are well developed, and ocelli may or may not be present. The forewings are somewhat longer than the hind wings, although the hind wings may be broader. Both pairs of wings and the body are usually covered with setae, or hairs, and occasionally with patches of scales. The wings are generally held rooflike when folded over the body. In most species the antennae are filamentous and about as long as the body, but in some families they can be several times longer than the body. Tibial spurs on the legs are conspicuous.
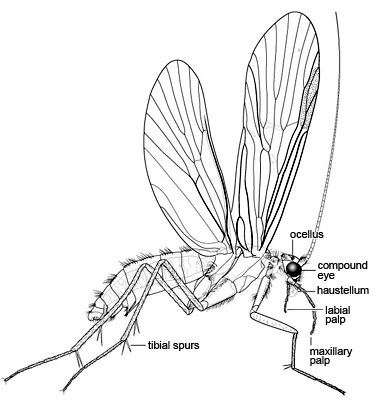
Larvae are aquatic and construct a case or retreat, except for a couple of "free-living" families. Like most holometabolous larvae, they have well developed mandibulate mouthparts and the thoracic legs are well developed, but abdominal prolegs are absent except for a pair of anal prolegs on the last abdominal segment, each proleg bearing a strong "anal claw." The exarate pupae are also aquatic, and have dectitious mandibles in most families.

Left: Caddisfly adult (Spicipalpia: Hydrobiosidae). Illustration copyright © 2004, Lourdes Chamorro-Lacayo. Right: Caddisfly larva and case (Integripalpia: Odontoceridae). Illustration copyright © 2004, Lourdes Chamorro-Lacayo.
The monophyly of the order Trichoptera is very well established. Following is a list of some characters that have been proposed as synapomorphic for the order (Weaver 1984, Kristensen 1991):
- Larvae aquatic, apneustic (no open spiracles), respiration epidermal, often by filamentous abdominal gills
- Larval tentorium reduced, delicate
- Larval antennae greatly reduced
- Larval abdominal segments I-IX without prolegs
- Larval abdominal segment IX with dorsal tergite
- Adult mandibles reduced, with loss of mandibular articulation
- Adult prelabium joined with hypopharynx to form a unique "haustellum" which serves as a lapping/sponging organ
Discussion of Phylogenetic Relationships
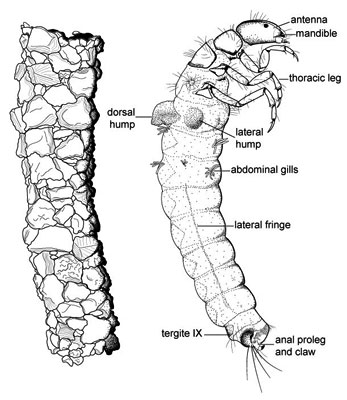
There has been considerable disagreement about the basal relationships of Trichoptera. This has resulted not only in different hypotheses about the evolutionary history of the group, but also in a confusion in the use of taxonomic categories, since different authors use different terminology, or have been inconsistent in how certain taxonomic categories have been used. In general, three major groups have been recognized, more or less corresponding to the different ecological adaptations of the larvae. We refer to these by their most common subordinal names, Annulipalpia, Spicipalpia, and Integripalpia (each in its most restricted sense and as used by Wiggins and Wichard 1989). However, the respective superordinal names of Hydropsychoidea, Rhyacophiloidea, and Limnephiloidea, respectively (sensu Ross 1956, Neboiss 1991), have sometimes been used to refer to groups of equivalent taxonomic coverage.
Because life history strategies have been used in constructing theories of caddisfly phylogenetic relationships, it is useful to review the life histories of the major groups:
- The Annulipalpia includes all of the families whose larvae make retreats and capture nets.
- The Spicipalpia includes several rather different groups, each with different larval habits:
- free-living and predaceous (Rhyacophilidae and Hydrobiosidae) - build no larval structures, but pupate within a domelike enclosure of mineral fragments.
- purse-case makers (Hydroptilidae) - free-living until the last larval instar, and then construct a case which is portable or cemented to the substrate and in which the larvae eventually pupate.
- tortoise-case or saddle-case makers (Glossosomatidae) - make a case that is constructed very much like the pupal cases of Annulipalpia and the free-living Spicipalpia, consisting of a dome of small sand grains and pebbles. However (to complete the analogy to a tortoise) the larva also makes a transverse strap beneath the dome, allowing the larva to carry it on its back. The tortoise-case makers construct a new and bigger case with each larval instar, and then pupate within the last larval case, after removing the ventral strap and attaching the case to the substrate.
- The Integripalpia have been called the tube-case makers, because they most commonly construct a tubular case. The case, however, can be made from very different materials or formed in peculiar ways in various species. The larvae are mobile and simply extend or add to the case with each larval instar, eventually pupating inside the slightly modified larval case.
There is broad agreement about the monophyly of two of these major taxonomic groups, the Annulipalpia and Integripalpia (as defined above), and considerable disagreement about the monophyly and relative placement of taxa within the Spicipalpia.
Morse (1997) provided a thorough summary of the hypotheses of relationships proposed for the major groups of Trichoptera and also of the status of phylogenetic work at the family and genus level undertaken to date. Ross (1956) was the first to identify explicitly any derived characters for caddisfly taxa. He recognized two monophyletic suborders, Annulipalpia (equivalent to his superfamily Hydropsychoidea) and Integripalpia. These subordinal names were first established by Martynov (1924). Ross's Integripalpia contained two superfamilies, Rhyacophiloidea and Limnephiloidea. These superfamilies are equivalent to the suborders Spicipalpia and Integripalpia, as used here. However, Ross's Rhyacophiloidea (Spicipalpia) was paraphyletic as originally defined. Further, his hypothesis of the relationships among the three rhyacophiloid families (Rhyacophilidae [including Hydrobiosidae], Glossosomatidae, and Hydroptilidae) and the Limnephiloidea was based mainly on a presumed evolutionary transformation in larval case/pupal cocoon making behavior. Ross lacked morphological synapomorphies at his Glossosomatidae + Hydroptilidae + Limnephiloidea node and had only one at his Hydroptilidae + Limnephiloidea node. Thus his behavioral transformation series is supported by only one non-behavioral character.
Weaver (1983, 1984, 1992a,b; also Weaver & Morse, 1986) was the first to apply cladistic principles to caddisfly higher level classification and examined about twice as many morphological characters as Ross. Weaver (1984) concluded that Spicipalpia (which he treated as an infraorder within a more broadly defined suborder Annulipalpia) was monophyletic and had a sister taxon relationship to the infraorder Curvipalpia (= Annulipalpia of Ross and as used here). Weaver restricted his concept of Integripalpia to include only the Limnephiloidea of Ross, and this is the sense in which it is used here.



Left: Tree of Weaver (1984). Right: Tree of Wiggins and Wichard (1989)
Later, Wiggins and Wichard (1989; also Wiggins 1992, Wichard 1991, Wichard et al. 1993, Wichard et al. 1997) proposed a sister group relationship between Annulipalpia and Integripalpia, based on an interpretation of pupal cocoon evolution in Trichoptera. Their phylogeny is based on the hypothesis that the closed, semipermeable cocoons of parchmentlike silk found in the spicipalpian families represent the groundplan condition of the order, and the cocoons of permeable silk with ventilation openings found in the Annulipalpia and Integripalpia are derived. A more detailed account of behavioral evolution in Trichoptera is found in the treatise of Frania and Wiggins (1997), who hypothesized that the ancestral habitat for the order Trichoptera was in cool, flowing, well-oxygenated water. This can be contrasted with the theory of Weaver and Morse (1986), who proposed that the ancestral trichopteran habitat was in subterranean silk-lined tubes in saturated soil. However, Kristensen (1997), pointed out that silk-lined tubes in Lepidoptera do not appear until the eight branch from the base of the tree, in Neolepidoptera (Exoporia), and thus this adaptation cannot be assumed to be ancestral within Trichoptera. Based on their hypothesis of evolution in the group, Wiggins, Wichard, and Frania would elevate Weaver's Infraorder Spicipalpia to a third suborder, coordinate with Annulipalpia and Integripalpia. According to Wiggins (1992), the pupation hypothesis was not intended as a statement of higher level trichopteran relationships; the recognition of Spicipalpia was one of convenience, since it serves to focus discussion on the unresolved problem of basal relationships in Trichoptera. These workers preferred to consider the relationships of the three suborders as currently unresolved.
In the study by Frania and Wiggins (1997), the hypothesis of a basal Spicipalpia was tested using a computer assisted parsimony analysis of 70 morphological larval and adult characters, which included the majority of families in the order. Characters were polarized using a hypothetical caddisfly ancestor, whose character states were inferred through consideration of character states in Mecoptera and Lepidoptera. The results could be considered equivocal at best, since they do not support either monophyly of Spicipalpia or of a basal position of Spicipalpia. The strict consensus phylogeny most closely resembles the hypothesis of Ivanov (1997, 2002):
Ivanov proposed that Hydroptilidae and Glossosomatidae of the Spicipalpia are sister taxa and allied to the Integripalpia, and that Rhyacophilidae and Hydrobiosidae are sister taxa allied to the Annulipalpia. He specifically challenged Weaver's hypothesis of spicipalpian monophyly, providing evidence that each of Weaver's 4 spicipalpian apomorphies are plesiomorphic. Frania and Wiggins similarly found Hydroptilidae to be closely related to Integripalpia, but the relationship of Glossosomatidae was less certain, depending on the analysis performed. Using selected characters they suggested that Glossosomatidae might be allied to Hydrobiosidae and Rhyacophilidae and basal to Annulipalpia, but were uncertain about the relationship of Hydroptilidae. In the cladograms presented, it was sister taxon to the Integripalpia, suggesting that Spicipalpia may not be monophyletic.
The most recent and complete analysis of basal relationships in Trichoptera is that of Kjer et al. (2001, 2002), who used a molecular dataset of several gene fragments, including mitochondrial, and nuclear DNA, and also included the morphological characters of Frania and Wiggins. Forty-three of forty-five families were included, and both parsimony and likelihood analyses were performed. This combined data analysis is the hypothesis of relationships presented above. In Kjer et al.'s analysis, Annulipalpia and Integripalpia were monophyletic and Spicipalpia was paraphyletically arranged at the base of the Integripalpia, as in Ross's hypothesis. The relationship of spicipalpians to Integripalpia was strongly supported, and Kjer et al. (2001, 2002) rejected the separation of some families of spicipalpians with Annulipalpia, as in the Ivanov (1997, 2002) and Frania and Wiggins (1997) morphology-based hypotheses. In fact, a differentially weighted analysis performed in Kjer et al. (2001) of the Frania and Wiggins (1997) morphological data recovered a phylogenetic hypothesis that was identical to Ross (1967). However, paraphyly of Spicipalpia was only weakly supported and the possibility that Spicipalpia is monophyletic could not be eliminated. Some partitions of the data supported a monophyletic Spicipalpia, and by most analytical criteria, a monophyletic Spicipalpia was only slightly suboptimal.
Determining the relative relationships among the families of Spicipalpia remains the next challenging question in resolving basal relationships in Trichoptera and in helping to resolve the question of the evolution of net and case-making behavior in the order.
Other Names for Trichoptera
- caddisworms
- caddis flies
- caddices
- Köcherfliegen
- frigáneas
- tricópteros
- trichopterans
- Caddisflies
References
Dohet, A. 2002. Are caddisflies an ideal group for the biological assessment of water quality in streams? Nova Supplementa Entomologica (Proceedings of the 10th International Symposium on Trichoptera) 15: 507-520.
Frania, H.E. and G.B. Wiggins. 1997. Analysis of morphological and behavioural evidence for the phylogeny and higher classification of Trichoptera (Insecta). Royal Ontario Museum Life Sciences Contributions 160. Ontario.
Friedlander, M. 1993. Phylogenetic position of rhyacophiloid caddisflies (Insecta, Trichoptera), a spermatological analysis of Rhyacophilidae and Glossosomatidae. Zoologica Scripta, 22(3): 299-304.
Friedlander, M. and R.E. Jeger. 1990. Phylogenesis of spermatogenesis in Annulipalpia caddisflies: An ultrastructural analysis on Philopotamidae spermiogenesis. Journal Of Structural Biology 105(1-3): 75-79.
Gall, W.K. 1994. Phylogenetic studies in the Limnephiloidea, with a revision of the world genera of Goeridae (Trichoptera). Ph.D. dissertation, Univ. of Toronto, Toronto.
Hickin, N.E. 1967. Caddis Larvae. Hutchinson. London.
Ivanov, V.D. 1997. Rhyacophiloidea: a paraphyletic taxon. Pages 189-193 in R.W. Holzenthal & O.S. Flint, Jr. (editors). Proceedings of the 8th International Symposium on Trichoptera. Ohio Biological Survey. Columbus.
Ivanov, V.D. 2002. Contribution to the Trichoptera phylogeny: new family tree with consideration of Trichoptera-Lepidoptera relations. Pages 277-292 in W. Mey (editor). Proceedings of the 10th International Symposium on Trichoptera. Nova Supplementa Entomologica, Keltern.
Ivanov, V.D. and M.V. Kozlov. 1987. Comparative analysis of pterothoracic musculature of caddis-flies (Insecta, Trichoptera). Zoologicheskii Zhurnal 66(10): 1484-1498.
Kjer, K.M., R.J. Blahnik, and R.W. Holzenthal. 2001. Phylogeny of Trichoptera (Caddisflies): Characterization of Signal and Noise Within Multiple Datasets. Systematic Biology 50(6): 781-816.
Kjer, K.M, R.J. Blahnik, and R.W. Holzenthal. 2002. Phylogeny of caddisflies (Insecta, Trichoptera). Zoologica Scripta, 31: 83-91.
Kobayashi, Y. and H. Ando. 1988. Phylogenetic relationships among the lepidopteran and trichopteran suborders (Insecta) from the embryological standpoint. Zeitschrift Fuer Zoologische Systematik Und Evolutionsforschung 26(3): 186-210.
Kristensen, N.P. 1984. Studies on the morphology and systematics of primitive Lepidoptera (Insecta). Steenstrupia 10: 141-191.
Kristensen, N.P. 1991. Phylogeny of extant hexapods. Pages 125-140 in CSIRO (editors). The Insects of Australia. Cornell University Press. Ithaca.
Kristensen, N.P. 1997. Early Evolution of the Lepidoptera + Trichoptera Lineage: Phylogeny and the Ecological Scenario. In Grandcolas, P. (editor), The Origin of Biodiversity in Insects: Phylogenetic Tests of Evolutionary Scenarios. Mém. Mus. Natn. Hist. Nat., 173: 253-271. Paris
Mackay, R.J. & G.B. Wiggins. 1978. Ecological diversity in the Trichoptera. Annual Review of Entomology 24: 185-208.
Malicky H. 1973. Trichoptera (Köcherfliegen). Handbuch der Zoologie, Bank IV, Halfte 2, Teil 2/29. Walter de Gruyter. Berlin.
Martynov, A.V. 1924. Rucheiniki (caddisflies [Trichptera]), pp. iv + 384. In, N.N. Bogdanova-Kat'kova [ed.], Prakticheskaya entomologiya, Volume 5. Leningrad.
Merritt, R.W. & K.W. Cummins. 1996. An introduction to the aquatic insects of North America, 3rd ed. Kendall/Hunt. Dubuque.
Morse, 1997. Phylogeny of Trichoptera. Annual Review of Entomology 42: 427-50.
Neboiss, A. 1991. Chapter 40. Trichoptera (Caddis-flies, caddises). Pages 787-816 in CSIRO (editors). The Insects of Australia. Cornell University Press. Ithaca.
Resh, V. H. 1993. Recent trends in the use of Trichoptera in water quality monitoring. Pages 285-291 in Proceedings of the 7th International Symposium on Trichoptera (C. Otto, ed.). Backhuys Publishers, Leiden, The Netherlands.
Resh, V.H. & D.M. Rosenberg (editors). 1984. The ecology of aquatic insects. Praeger, New York.
Resh, V.H. & J.D. Unzicker. 1975. Water quality monitoring and aquatic organisms: the importance of species. identification. Journal Water Pollution Control Federation, Washington 47(1): 9-19.
Rosenberg, D.M. & V.H. Resh (editors). 1993. Freshwater Biomonitoring and Benthic Macroinvertebrates. Chapman and Hall. New York
Ross, H.H. 1944. The caddisflies or Trichoptera of Illinois. Bulletin of the Illinois Natural History Survey 23(1) 1-326.
Ross, H.H. 1956. Evolution and classification of the mountain caddisflies. University of Illinois Press, Urbana.
Ross, H.H. 1964. Evolution of caddis worm cases and nets. American Zoologist, 4: 209-220.
Ross, H.H. 1967. The evolution and past dispersal of the Trichoptera. Annual Review of Entomology 12: 169-206.
Schmid, F. 1980. Genera des Trichoptères de Canada et des États adjacents. Les Insectes et Arachnides du Canada, part 7. Agriculture Canada, Publication 1692. Ottawa.
Schmid, F. 1984. Un essai d'évaluation de la faune mondiale des Trichoptères. Page 337 in J.C. Morse (editor). Proceedings of the 4th International Symposium on Trichoptera. Dr. W. Junk, Publishers. The Hague.
Scott, K.M.F. 1993. Three recently erected Trichoptera families from South Africa, the Hydrosalpingidae, Petrothrincidae and Barbarochthonidae (Integripalpia: Serocostomatoidea), with a cladistic analysis of character states in the twelve families here considered as belonging to the Sericostomatoidea, by F.C. de Moor. Annals of the Cape Provincial Museums (Natural History), 18(14): 293-354.
Shields, O. 1988. Mesozoic history and neontology of Lepidoptera in relation to Trichoptera, Mecoptera, and angiosperms. Journal Of Paleontology 62(2): 251-258.
Sukatcheva, L.D. 1991. Historical development of the order Trichoptera. Pages 441-445 in C. Tomaszewski (editor). Proceedings of the 6th International Symposium on Trichoptera. Adam Mickiewiscz University Press. Poznan, Poland.
Weaver, J.S., III. 1983. The evolution and classification of Trichoptera, with a revision of the Lepidostomatidae and a North American synopsis of this family. Ph.D. dissertation, Clemson Univ. Clemson, South Carolina.
Weaver, J.S., III. 1984. The evolution and classification of Trichoptera, Part 1: the groundplan of Trichoptera. Pages 413-419 in J.C. Morse (editor). Proceedings of the 4th International Symposium on Trichoptera. Dr. W. Junk Publishers, The Hague.
Weaver, J.S., III. 1992a. Remarks on the evolution of Trichoptera: a critique of Wiggins and Wichard's classification. Cladistics 8: 171-180.
Weaver, J.S., III. 1992b. Further remarks on the evolution of Trichoptera: a reply to Wiggins. Cladistics 8: 187-190.
Weaver, J.S., III and H. Malicky. 1994. The genus Dipseudopsis Walker from Asia (Trichoptera: Dipseudopsidae). Tijdschrift voor Entomologie, 137: 95-142.
Weaver, J.S. III & J.C. Morse. 1986. Evolution of feeding and case-making behavior in Trichoptera. Journal of the North American Benthological Society 5(2): 150-158.
Wheeler, W.C, M. Whiting, Q.D. Wheeler, and J.M. Carpenter. 2001. The phylogeny of extant hexapod orders. Cladistics 17: 113-169.
Wichard, W., H.P. Klein, & P. Herter. 1997. Pupal cocoon of Amphiesmenoptera (Lepidopera = Trichoptera) with evolutionary considerations of the Trichoptera. Pages xx-xx. In: R.W. Holzenthal & O.S. Flint, Jr. (editors). Proceedings of the 8th International Symposium on Trichoptera. Ohio Biological Survey. Columbus.
Wiggins, G.B. 1992. Comments on the phylogeny of pupation behavior in Trichoptera: a response to Weaver. Cladistics 8: 181-185. Wiggins, G.B. 1996. Larvae of the North American caddisfly genera (Trichoptera), 2nd ed. University of Toronto Press, Toronto.
Wiggins, G.B. & W.K. Gall. 1993. The Asian caddisfly family Phryganopsychidae: phylogenetic novelty or relict?, pp. 149-154. In, C. Otto [ed.], Proceedings of the 7th International Symposium on Trichoptera. Backhuys Publishers. Leiden.
Wiggins, G.B. & W. Wichard. 1989. Phylogeny of pupation in Trichoptera, with proposals on the origin and higher classification of the order. Journal of the North American Benthological Society 8(3): 260-276.
Williams, D. D., A. F. Tavares, and E. Bryant. 1987. Respiratory device or camouflage? A case for the caddisfly. Oikos 50(1): 42-52.
Information on the Internet
- World Trichoptera Checklist, Dr. John C. Morse (editor), Clemson University
- Orden Trichoptera, INBio, Insect Families of Costa Rica (in Spanish)
- TRICH-L, the Trichoptera discussion group list. To subscribe send the following message IN THE BODY ONLY:
subscribe Trich-L your name
to the following address:
cuentsubscribe@entoinfo.clemson.edu
About This Page
Ralph W. Holzenthal

University of Minnesota, St. Paul, Minnesota, USA
Roger J. Blahnik

University of Minnesota, St. Paul, Minnesota, USA
Aysha Prather

Centre for Biodiversity and Conservation Biology, Royal Ontario Museum, Toronto, Ontario, Canada
Karl Kjer

Rutgers University, New Brunswick, New Jersey, USA
Correspondence regarding this page should be directed to Ralph W. Holzenthal at
holze001@umn.edu
Page copyright © 2010 Ralph W. Holzenthal, Roger J. Blahnik, Aysha Prather, and Karl Kjer
 Page: Tree of Life
Trichoptera. Caddisflies.
Authored by
Ralph W. Holzenthal, Roger J. Blahnik, Aysha Prather, and Karl Kjer.
The TEXT of this page is licensed under the
Creative Commons Attribution-NonCommercial-ShareAlike License - Version 3.0. Note that images and other media
featured on this page are each governed by their own license, and they may or may not be available
for reuse. Click on an image or a media link to access the media data window, which provides the
relevant licensing information. For the general terms and conditions of ToL material reuse and
redistribution, please see the Tree of Life Copyright
Policies.
Page: Tree of Life
Trichoptera. Caddisflies.
Authored by
Ralph W. Holzenthal, Roger J. Blahnik, Aysha Prather, and Karl Kjer.
The TEXT of this page is licensed under the
Creative Commons Attribution-NonCommercial-ShareAlike License - Version 3.0. Note that images and other media
featured on this page are each governed by their own license, and they may or may not be available
for reuse. Click on an image or a media link to access the media data window, which provides the
relevant licensing information. For the general terms and conditions of ToL material reuse and
redistribution, please see the Tree of Life Copyright
Policies.
- First online 14 February 1997
- Content changed 20 July 2010
Citing this page:
Holzenthal, Ralph W., Roger J. Blahnik, Aysha Prather, and Karl Kjer. 2010. Trichoptera. Caddisflies. Version 20 July 2010 (under construction). http://tolweb.org/Trichoptera/8230/2010.07.20 in The Tree of Life Web Project, http://tolweb.org/









 Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site
Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site