Strepsiptera
Twisted-wing parasites
Jeyaraney Kathirithamby

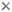
This tree diagram shows the relationships between several groups of organisms.
The root of the current tree connects the organisms featured in this tree to their containing group and the rest of the Tree of Life. The basal branching point in the tree represents the ancestor of the other groups in the tree. This ancestor diversified over time into several descendent subgroups, which are represented as internal nodes and terminal taxa to the right.

You can click on the root to travel down the Tree of Life all the way to the root of all Life, and you can click on the names of descendent subgroups to travel up the Tree of Life all the way to individual species.
For more information on ToL tree formatting, please see Interpreting the Tree or Classification. To learn more about phylogenetic trees, please visit our Phylogenetic Biology pages.
close boxIntroduction
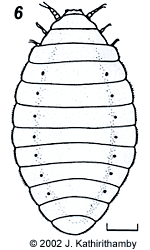
Strepsiptera are obligate parasites of insects, with hosts ranging across 7 orders and 34 families. The name of the group is derived from the Greek words for twisted (streptos) and wing (pteron) and refers to the peculiar twisted wing of the male's hind-wings while in flight. Representatives of the suborder Mengenillidia generally show more primitive characteristics (fig. 1). The Mengenillidae parasitize Thysanura (Lepismatidae), the only known order in the sub-class Apterygota to be attacked by strepsipterans, while Mengeidae are known only from fossil males from Baltic amber. We have very little information about their life history, therefore.
Strepsiptera exhibit extreme sexual dimorphism, which is most pronounced in the suborder Stylopidia. Strepsipteran males emerge from the host after endoparasitic pupation in the host. Adult males are free-living, and their sole mission is to find and fertilize a female. They have reduced forewings and fan-shaped hind wings, branched antennae, and raspberry-like eyes (title image and fig. 2); the latter are very unusual among living insects and form a modern counterpart to the structural plan proposed for eyes of trilobites (Kinzelbach 1971, 1990, Kathirithamby 1989, Buschbeck et al. 1999).
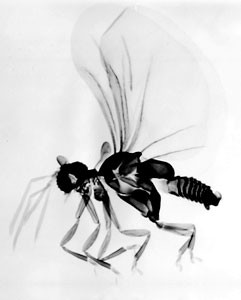
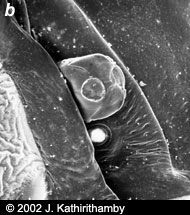
Figure 3. Cephalothorax of adult females. Micrographs copyright © 2002 Kathirithamby
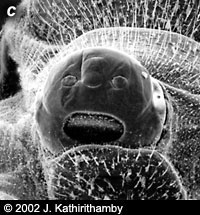
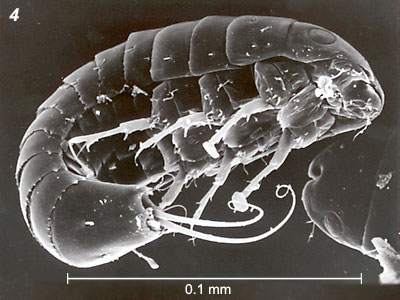
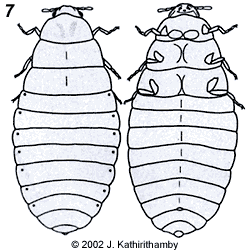
Females of the family Stylopidae are neotenic (i.e., they retain juvenile features even in adulthood) and totally endoparasitic in their hosts. They are highly modified morphologically, lacking adult external characteristics such as eyes, antennae, legs, wings and external genitalia (fig. 3). Apart from the adult males, the only free-living stages in this suborder are the viviparous 1st instar host-seeking larvae (fig 4). In contrast, males and females in the family Mengenillidae leave the host at the end of the last larval instar to pupate externally (fig. 5, 6). After eclosion, the females are free-living, with the presence of all other adult characteristics such as eyes, mouthparts, antennae, legs and a ventral genital opening, but with the absence of wings (fig. 7).
Figure 4. 1st instar free-living larva: Stichotrema dallatorreanum Hofeneder (Myrmecolacidae) (Papua New Guinea). From Kathirithamby & al. (1998). Copyright © 1998 Taylor & Francis.
Drawings after Kinzelbach 1971. Copyright © 2002 J. Kathirithamby
The combination of morphological reduction and modification, and the bizarre and unusual life history of Strepsiptera, have puzzled biologists for over two centuries (Rossi 1793; Latreille 1809; Kirby 1802, 1813. 1815; Lamark 1816 Pierce 1909; Crowson 1960, 1981; Arnett 1963; Kinzelbach 1971, 1990; Kathirithamby 1989), and the Strepsiptera's phylogenetic position has been the most enigmatic question in ordinal level insect systematics (the "Strepsiptera problem", Kristensen 1981, see below).
Strepsiptera are cosmopolitan in distribution (Table 1), but are extremely difficult to locate, and one often has to find the host in order to find the female. To date, 596 species of Strepsiptera have been described and many more await description. Many of the described species are of free-living males that have come into traps. At present, since sites have been located where Strepsiptera can be collected, some of the species are being reared in the laboratory. Work on the extraordinary reproductive and developmental biology and behavioural ecology of this group is under way.
Table 1: Geographical distribution of the extant families of Strepsiptera.
| Family | Geographical Range | ||||||
|---|---|---|---|---|---|---|---|
| Palaearctic | Afrotropical | Australian | Oriental | Neotropical | Nearctic | ||
| Mengenillidae | + | + | + | + | - | - | |
| Corioxenidae | + | + | + | + | + | + | |
| Halictophagidae | + | + | + | + | + | + | |
| Elenchidae | + | + | + | + | + | + | |
| Myrmecolacidae | + | + | + | + | + | + | |
| Stylopidae | + | + | + | + | + | + | |
| Bohartillidae | - | - | - | - | + | - | |
| Callipharixenidae | - | - | - | + | - | - | |
Characteristics
Strepsiptera are characterized by the following synapomorphies:- Free-living host-seeking 1st instar larvae, which are produced viviparously by the neotenic female. Several hundred are produced which emerge from the female and seek new hosts. Sexual dimorphism does not exist in the 1st instars. These 1st instars are often known as "triungulin" larvae. This term was initially applied to the Meloidae, because of the presence of three claws on the legs. The term was later extended to the Rhipiphoridae and Strepsiptera, and refers to the active host-seeking larvae. However, morphologically, the 1st instar larvae of Strepsiptera do not resemble the Meloidae. The pulvillus of the 1st pair of legs is disk-like, and slender; single, spine-like tarsi are present on the 2nd and 3rd pair of legs, while there is absence of claws. In addition, the 1st instars have highly serrated tergites and sternites, presumably to enable them to cling to the hosts and/or vegetation while awaiting entry. The head bears antennae, mandibles, and labrium, and the abdominal setae are long, and are about a third or half the body length.
- The 1st instar larvae moult, on entry into the host, to an apodous 2nd instar. Therefore, all Strepsiptera exhibit hypermetamorphosis (two morphologically distinct larval instars—the 1st larva and subsequent endoparasitic stages).
- The endoparasitic larvae undergo apolysis without ecdysis (Kathirithamby et al. 1984) whereby the larvae moult but do not shed the old cuticle. It was found that Elenchus tenuicornis (Kirby) and Stichotrema dallatorreanum Hofeneder have four larval instars (Kathirithamby 1998).
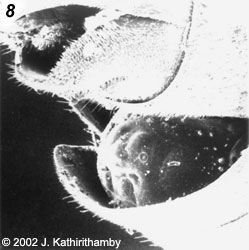
- In the male, at the last larval instar, the cuticle is sclerotized to form the puparium (fig. 8) (Kathirithamby 1983).
Images copyright © 2002 J. Kathirithamby
- The free-living males have prominent branched antennae and raspberry-like eyes, reduced forewings and large hindwings (title image and fig. 2). They are short-lived, and their sole mission on emergence from the host (fig. 9) is to find and fertilize a female. There is no trochanter on the fore and middle legs, and the metacoxae are fused to the pleurosternum. The male has an aedeagus but no copulatory apparatus, such as parameres.
- The females are neotenic (fig. 3) and totally endoparasitic in their hosts (except in the family Mengenillidae). The external cuticle on the ventral surface is analogous to the peritrophic membrane (found in the midgut of other insects) in adult females (Kathirithamby 2000). The whole abdominal cavity of the adult female is filled with developing embryos. Fertilization is by haemocoelic insemination, and reproduction is by haemocoelous vivipary.
Discussion of Phylogenetic Relationships
Kinzelbach (1971) divided the Strepsiptera into two suborders (Mengeillidia and Stylopidia) and nine families. Seven of these families are here united in the suborder Stylopidia, which is defined by many shared derived characteristics. The monophyly of this group has recently also been confirmed by Pohl's (2002) analysis of the 1st instar larvae of nearly all extant strepsipteran families.
The family Mengeidae is known only from a few fossil males from Baltic amber, all representing a single species, Mengea tertiaria (Menge). Mengea shares many plesiomorphic characters with the Mengenillidae, such as:
- Similar round head capsule
- Inwardly directed mandibles
- Presence of abdominal stigmata
- 5-jointed tarsi
- Straight aedeagus.
Therefore, the two groups have often been associated with one another (Pierce 1908, 1909, 1918; Ulrich 1927, 1956; Bohart 1941, Luna de Carvalho 1956, 1959 1961, 1967; Kinzelbach 1969a; Riek 1970). Since all of the traits shared by Mengea and the Mengenillidae may be plesiomorphic, and there are a few tentative morphological similarities between Mengea and the stylopidian family Corioxenidae, Kinzelbach (1971)included the Mengeidae in the Stylopidia. However, in 1978 Kinzelbach placed the Mengeidae in the Mengenillidia. He says the formation of the labrium, free metacoxa, number of antennal joints, wing vein CuP are more plesiomorphic than the Mengenillidae, and the recent Mengenillidae may be thought to be derived from Mengeidae. Since females are unknown it will be regarded as a sister group of the Mengenillidae. Hence the phylogenetic position of the Mengeidae will remain uncertain until additional specimens are found which allow a more detailed analysis of the morphology and life history of this fossil group.
Relationship of Strepsipterans to Other Insects
Strepsiptera are a monophyletic group (Henning 1981). In recent years, four phylogenetic placements of these insects have been proposed:- Sister group to the Endopterygota. Kristensen (1991, 1995) noted that the position of Strepsiptera may not be within Endopterygota since:
- the pupal stage is preceded by a couple of pharate instars (Whiting et al. 1997 interpreted this as the 2nd instar) with external wing buds
- larval eyes are carried over to the adult stage
- Crowson (1960, 1981) placed Strepsiptera within the coleopteran suborder Polyphaga, as sister to Rhipiphoridae. This theory was based on derived features in rhipiphorids which he said were similar to Strepsiptera:
- active host-seeking 1st instar larva
- hypermetamorphosis from a 1st instar larva to apodous endoparasitic larvae
- flabellate antennae
- reduced forewings (in some genera)
- active host-seeking stage and hypermetamorphosis have arisen once in the Exopterygota and five times in the endopterygota;
- flabellate antennae are found in many insects;
- as pointed out by Kathirithamby (1989) and Pix et al. (1993), the reduced forewings in Strepsiptera are neither morphologically nor functionally similar to the elytron in Coleoptera.
- Handlirsch (1903), Boerner (1904) and Shipley (1904) placed Strepsiptera as a sister group to Coleoptera, and Kinzelbach (1971, 1990) and Kathirithamby (1989, 1991) argued that this placement was based on only one character: posteromotorism (use of the hind wings for flight). The venational characters supporting the sister group relationship between the Coleoptera and Strepsiptera (Kukalova-Peck and Lawrence 1993) were disputed by Whiting and Kathirithamby (1995).
- The hypothesis that the Strepsiptera are a sister group to true flies (Diptera) is based on both morphological and molecular evidence, and has been championed by Whiting and Wheeler (1994) and Whiting et al. (1997), but remains controversial.
- Whiting and Wheeler (1994) published a short note indicating that a phylogenetic analysis of 18S ribosomal DNA suggested that Strepsiptera were related to Diptera. No data or details of the analysis were included in the note, but this information eventually appeared in Whiting et al. 1997. The authors further suggested that the reduced, mesothoracic wings of strepsipteran males may be homologous to the halteres (on the metathorax) of dipterans, and that their presence on different thoracic segments is due to homeotic mutation.
- Carmean and Crespi (1995), in response, provided an analysis of 13 18S ribosomal DNA sequences, and showed that the branches leading to Diptera and Strepsiptera were both very long. They suggested that the grouping of these taxa in a parsimony analysis of the data might be artifactual, caused by long-branch attraction (Felsenstein 1978).
- Chalwatzis et al. (1996) analysed 18S rDNA of 19 insect species, and similarly found a grouping of Strepsiptera plus Diptera, even though they used distance methods (with several distance measures) that would be affected by long-branch attraction under different conditions than parsimony methods.
- Whiting et al. (1997) presented their complete analysis, using parsimony methods, of 85 18S rDNA sequences and 52 28S rDNA sequences, as well as morphological data. This analysis also supported a grouping of Strepsiptera plus Diptera.
- Huelsenbeck (1997) showed that the data set of 13 18S ribosomal DNA sequences used by Carmean and Crespi (1995), when analysed with maximum likelihood methods, yielded a tree with Strepsiptera as sister to Coleoptera. He suggested that long branch attraction is the cause of the placement of Strepsiptera with Diptera when parsimony methods are used.
- Rokas et al. (1999) investigated the potential of an intron insertion site as a phylogenetic character. They found that the en homeobox gene of Stichotrema dallatorreanum lacks the derived intron insertion shared by representatives of Diptera and Lepidoptera. They thus argued against a close affiliation between Strepsiptera and Diptera.
- Huelsenbeck (2001) observed that quality and quantity of morphological data available are limited, and that it is molecular data which hold the promise to solving the problem. The two ribosomal genes available, 18S and 28S, produced different placements of Strepsiptera. He states that more molecular data are needed to solve the phylogenetic placement of Strepsiptera.
Therefore the position of Strepsiptera among Insecta is still not solved, but work is being carried out on molecular and morphological data to try and resolve the question of the placement of Strepsiptera. Collaborating laboratories are: Jeyaraney Kathirithamby and Peter Holland (Oxford), John Huelsenbeck (San Diego) and Spencer Johnston (Texas A&M).
References
Boerner, C. 1904. Zur Systematik der Hexapoda (Strepsiptera). Zool. Anz. 27: 511-533.
Bohart, R. M. 1941. A revision of the Strepsiptera with special reference to the species of North America. Univ. Calif. Publs. Ent. 7: 91-160.
Buschbeck, E., B. Ehmer and Hoy, R. 1999. Chunk versus point sampling: visual imaging in a small insect. Science 286: 1178-1180.
Carmean, C. and B.J. Crespi. 1995. Do long branches attract flies? Nature 373: 666.
Chalwatzis, N., Bauf, J., van De Peer, Y., Kinzelbach, R. & Zimmermann, F.K. (1996) 18S ribosomal RNA genes of insects: Primary structure of the genes and molecular phylogeny of the Holometabola. Annals of the Entomological Society of America, 89: 788-803.
Crowson, R. A. 1960. The phylogeny of Coleoptera. Annual Review of Entomology, 5: 111-134.
Crowson, R.A. 1981. The Biology of the Coleoptera. Academic Press, New York.
Felsenstein, J. 1978. Cases in which parsimony or compatibility methods will be positively misleading. Systematic Zoology 27:401-410.
Handlirsch, A 1903 Zur Phylogenie der Hexapoden. Sitzungsberichte der Akademie der Wissenschaften, Wien 113: 716-738.
Hennig, W. 1981. Insect Phylogeny. Academic Press, New York.
Huelsenbeck, J.P. 1997. Is the Felsenstein Zone a fly trap? Systematic Biology 46:69-74.
Huelsenbeck, J. P. 2001. A Bayesian perspective of the Strepsiptera problem. Tidjschrift voor Entomologie 144: 165-178.
Kathirithamby, J. 1989. Review of the order Strepsiptera. Systematic Entomology 14: 41-92.
Kathirithamby, J. 1991. Strepsiptera. Pp. 684-695 in The Insects of Australia: A textbook for students and research workers, 2nd edition (I. D. Naumann, P. B. Carne, J. F. Lawrence, E. S. Nielsen, J. P. Spradberry, R. W. Taylor, M. J. Whitten and M. J. Littlejohn eds.). CSIRO, Melbourne Univ. Press, Melbourne.
Kathirithamby, J. 1993. Myrmecolacidae (Strepsiptera) from Australia. Invertebrate Taxonomy 7:859-873.
Kathirithamby, J. 2000. Morphology of the female Myrmecolacidae (Strepsiptera) including the apron, and an associated structure analogous to the peritrophic matrix. Zoological Journal of the Linnean Society 128: 269-287.
Kathirithamby, J. 2009. Host-parasitoid associations in Strepsiptera. Annual Review of Entomology 54:227 - 249.
Kathirithamby, J., S. Simpson, T. Solulu and R. Caudwell. 1998. Strepsiptera parasites - novel biocontrol tools for oil palm integrated pest management in Papua New Guinea. International Journal of Pest Management 44: 127-133.
Kathirithamby, J., D. S. Spencer Smith, M. B. Lomas and B. M. Luke. 1984. Apolysis without ecdysis in larval development of a strepsipteran, Elenchus tenuicornis. Zoological Journal of the Linnean Society. 82: 335-343.
Kinzelbach, R. 1969. 78. Familie: Stylopidae, Fächerflügler (=Ordnung Strepsiptera).- In: Harde, J. W., H. Freude & G. A. Lohse. Die Käfer Mitteleuropas 8: 136-159.
Kinzelbach, R. K. 1971. Morphologische Befunde an Fächerflüglern und ihre phylogenetische Bedeutung (Insecta: Strepsiptera). Zoologica 119(1&2) pp. 256.
Kinzelbach, R.K. 1978. Strepsiptera. Die Tierwelt Deutschlands 65: 166pp.
Kinzelbach, R. 1990. The systematic position of Strepsiptera (Insecta). American Entomologist 36:292-303.
Kristensen, N.P. 1991. Phylogeny of extant hexapods. Pp. 125-140 in The Insects of Australia: A textbook for students and research workers, 2nd edition (I. D. Naumann, P. B. Cornell Carne, J. F. Lawrence, E. S. Neilson, J. P. Spradberry, R. W. Taylor, M. J. Whitten and M. J. Littlejohn eds.). CSIRO, Melbourne Univ. Press, Melbourne. Ithaca, New York.
Kristensen, N.P. 1995. Forty years' insect phylogenetics systematics. Zoologische Beiträge. N.F. 36: 83-124.
Kukalova-Peck, J. and J.F. Lawrence. 1993. Evolution of the hind wing in Coleoptera. Canadian Entomologist 125: 181-258.
Luna de Carvalho, E. 1956. Primeria contribuição para o Estudo dos Estrepsipteros angloenses (Insecta, Strepsiptera). Publicações Culturais da Campanhia de Diamanies de Angola 29: 11-54.
Luna de Carvalho, E. 1959. Segunda contribuição para o Estudo dos Estrepsipteros angloenses (Insecta, Strepsiptera). Publicações Culturais da Campanhia de Diamanies de Angola 41: 125-154;
Luna de Carvalho, E. 1961. Tabela para a determinação dos generos de Estrepsipteros (Insecta). Gracia de Orta 9: 691-698. Garcia de Orta 9: 691-698.
Luna de Carvalho, E. 1967. Terçeira contribuição para o Estudo dos Estrepsipteros angloenses (Insecta, Strepsiptera). Publicações Culturais da Campanhia de Diamanies de Angola 77: 13-66.
Pierce, W. D. 1908. A preliminary review of the classification of Strepsiptera. Proceedings of the Entomological Society of Washington. 9: 75-85.
Pierce, W. D. 1909. A monographic revision of the twisted winged insects comprising the order Strepsiptera Kirby. Bulletin of the United States National Museum 66: 1-232.
Pierce, W.D. 1964. The Strepsiptera are a true order, unrelated to Coleoptera. Annals of the Entomological Society of America 57: 603-605.
Pix, W., G. Nalbach, J. and Zeil. 1993. Strepsipteran forewings are haltere-like organs of equilibrium. Naturwissenschaften 80: 371-374.
Pohl, H. 2002. Phylogeny of the Strepsiptera based on morphological data of the first instar larvae. Zoologica Scripta 31:123-138.
Pohl, H. and R. G. Beutel. 2005. The phylogeny of Strepsiptera (Hexapoda). Cladistics 21:328-374.
Pohl, H., R. G. Beutel, and R. Kinzelbach. 2005. Protoxenidae fam. nov. (Insecta, Strepsiptera) from Baltic amber - a 'missing link' in strepsipteran phylogeny. Zoologica Scripta 34(1):57-69.
Rokas, A., J. Kathirithamby and P. W. H. Holland. 1999. Intron insertion as a phylogenetic character: the engrailed homeobox does not indicate affinity with Diptera. Insect Molecular Biology 8: 527-530.
Riek, E. 1970. Strepsiptera. Insects of Australia, 00. 622-635. University of Melbourne Press.
Shipley, A. E. 1904. The orders of insects (Strepsiptera). Zoologischer Anzeiger 27: 259-262.
Ulrich, W. 1927. Strepsiptera, Fächerflügler. – In: P. Schultz, Biologie der Tiere Deutschlands. 41; 10103.
Ulrich, W. 1956. Unsere Strepsipteren-Arbeiten. Zool. Beitr. N. F., 2:177.
Whiting, M.F. and J. Kathirithamby. 1995. Strepsiptera do not share hind wing venational synapomorphies with Coleoptera: a reply to Kukalova-Peck and Lawrence. Journal of the New York Entomological Society 103(1): 1-14.
Whiting, M.F. and W.C. Wheeler. 1994. Insect homeotic transformation. Nature 368: 696.
Whiting, M.F., J.C. Carpenter, Q.D. Wheeler and W.C. Wheeler. 1997. The Strepsiptera problem: Phylogeny of the holometabolous insect orders inferred from 18S and 28S ribosomal DNA sequences and morphology. Systematic Biology, 46: 1-68.
Information on the Internet
- Strepsiptera. Hans Pohl's Strepsiptera page with many great images.
- Systematics, Developmental Biology and Biodiversity of Strepsiptera (Insecta). Jeyaraney Kathirithamby, Oxford University.
- Checklist for Strepsiptera. Australian Faunal Directory.
- An insect with 100 eyes has left scientists, well, surprised. 1999 Scientific American exhibit about Buschbeck et al.'s research on the eyes of strepsipteran males.
About This Page
Jeyaraney Kathirithamby

University of Oxford, Oxford, UK
Correspondence regarding this page should be directed to Jeyaraney Kathirithamby at
jeyaraney.kathirithamby@zoology.oxford.ac.uk
Page copyright © 2002 Jeyaraney Kathirithamby
All Rights Reserved.
- First online 24 September 2002
Citing this page:
Kathirithamby, Jeyaraney. 2002. Strepsiptera. Twisted-wing parasites. Version 24 September 2002 (under construction). http://tolweb.org/Strepsiptera/8222/2002.09.24 in The Tree of Life Web Project, http://tolweb.org/








 Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site
Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site