Cercozoa
David Bass and Tom Cavalier-Smith

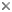
This tree diagram shows the relationships between several groups of organisms.
The root of the current tree connects the organisms featured in this tree to their containing group and the rest of the Tree of Life. The basal branching point in the tree represents the ancestor of the other groups in the tree. This ancestor diversified over time into several descendent subgroups, which are represented as internal nodes and terminal taxa to the right.

You can click on the root to travel down the Tree of Life all the way to the root of all Life, and you can click on the names of descendent subgroups to travel up the Tree of Life all the way to individual species.
For more information on ToL tree formatting, please see Interpreting the Tree or Classification. To learn more about phylogenetic trees, please visit our Phylogenetic Biology pages.
close boxIntroduction
Cercozoa are mostly heterotrophic protozoa dwelling abundantly in soil (where they are the most numerous eukaryotes) and in all freshwater and marine habitats. Some parasitize plants, invertebrate animals and other protists. A few have become algae by enslaving photosynthetic prey to form permanent cellular chimaeras. Free-living Cercozoa feed on bacteria, fungi, algae, other protozoa or even microscopic animals. They may be the most numerous predators on earth, but unlike most predators they generally lack a mouth as they can catch prey over much of their typically soft body surface.
Their ancestral body plan was a predatory, phagotrophic amoeboflagellate with one anterior and one posterior cilium. One lineage enslaved a green algal cell to become the chimaeric chlorarachnean algae, which still retain the nucleus (as a miniaturized nucleomorph), former plasma membrane (as the periplastid membrane), and chloroplast of the enslaved green alga. Cercozoa have diversified into so many distinct lineages that body plans have become extremely varied. Some have lost cilia altogether and become amoebae with thread-like (filose) or net-like (reticulose) pseudopodia for locomotion and prey entrapment. These filose or reticulose amoebae are either naked or have evolved rigid tests (like a shell), which happened independently for at least three groups (euglyphid, tectofilosid, and gromiid testate amoebae). Some have evolved radiating axopodia supported by microtubules to catch prey instead (Phaeodarea, desmothoracids) and thus mimic radiolaria or heliozoa. Others have abandoned pseudopodia and become pure flagellates, either gliding on surfaces or swimming in the plankton. Many have retained cilia (sometimes reduced to one or increased to four or more) and pseudopods. Cell surfaces of the flagellates are generally naked and quite soft, lacking the complex pellicles found in alveolates and excavates, but thaumatomonads have evolved a semi-rigid covering of complex silica scales, which still allows branching filopodia to be used for feeding and cilia to propel the cells by gliding.
Cercozoa were only recently recognised as a monophyletic group (Cavalier-Smith 1997) and were named Cercozoa in 1998 (Cavalier-Smith 1998). The name is based on the cercomonads, amoeboflagellates that are abundant in soil and freshwater, which were first discovered by Dujardin (1841) the father of protozoology, who first realised that protozoa were cells. Even earlier Dujardin (1835) had discovered the reticulose testate cercozoan amoeba Gromia. Cercomonad means ‘tailed monad’, because almost all cercomonads have an extensible pseudopodial tail that is typically drawn out along the posterior gliding cilium, sometimes obscuring it. Dujardin coined the name Rhizopoda (root feet) for organisms having filose or reticulose pseudopods. Unfortunately other authors later confusingly expanded that term to include also the unrelated Amoebozoa, which generally have broad lobose, non-root-like pseudopods. Cercozoa are now known to be one of the most diverse, speciose and ecologically important of all protozoan phyla and include the majority (not all) of eukaryotes with filose pseudopods or cilia that glide on surfaces instead of swimming (Cavalier-Smith and Chao 2003). Many lineages are currently known only from environmental DNA sequencing (Bass and Cavalier-Smith 2004; Bass et al. 2009), so cercozoan diversity must be even greater than is now appreciated.
Characteristics
Typically amoeboflagellates, flagellates or filose or reticulose amoebae, sometimes axopodial or parasitic. Most lineages have a strong propensity to form filopodia (often branching, with or without granular extrusomes) or reticulopodia or axopodia.
Most flagellates are not planktonic but glide on surfaces, e.g. soil particles, marine or freshwater sediments, plant stems or suspended particles of ‘marine snow’. They use their posterior cilium as a ski, which adheres closely to the substratum; gliding is probably driven by kinesin molecular motors attached to ciliary membrane proteins that kinesin can move within the fluid lipid membrane by walking along the outer ciliary doublet microtubules. One recently discovered deep-branching filosan lineage (still unnamed), uniquely in eukaryotes, uses both its cilia for surface gliding.
The ciliary transition region bears two characteristic structures absent from all other eukaryotes: the proximal hub-lattice and the distal nonagonal fibre (Cavalier-Smith et al. 2008). In most Cercozoa the ciliary transition region (which lies between where the centriolar triplets end and the ciliary centre pair begins) is very short, but in a few groups that constitute a single derived clade (together with related amoebae) it is rather long, and their nonagonal fibre is obscured by a dense upper transitional plate (Spongomonadida, Thaumatomonadida, Cryomonadida, Pansomonadida, Glissomonadida). The anterior cilium is younger, and the posterior cilium the mature one; thus ciliary transformation occurs over two cell cycles as in other bikont eukaryotes. Cilia typically lack scales (except for the Thaumatomastix anterior cilium) or hairs; simple non-tubular hairs are present in some Allas (anterior) and in Aurigamonas (posterior).
Cercozoa are typically unicellular and uninucleate and undergo binary fission. But some are multinucleate plasmodia (Plasmodiophorida, Ascetosporea, Filoreta) or can temporarily form such plasmodia (many cercomonads and thaumatomonads; some chlorarachneans). Some, notably Phaeodarea and Plasmodiophorida typically undergo multiple fission. Sex is certainly known only in plasmodiophorids. Cysts are widespread.
Extrusomes (typically simple and rounded; more elongate in thaumatomonads; very long in cryomonads) are widely present in filosan flagellates and in granofilosans, but not in other cercozoan amoebae.
Discussion of Phylogenetic Relationships
Cercozoa fall quite clearly into two major groups, which are probably sisters: Filosa and Endomyxa. Filosa include all the gliding flagellates and amoeboflagellates, as well as numerous amoebae with filose non-anastomosing pseudopods, plus all the cercozoan algae (chlorarachneans, plus the euglyphid filose amoeba Paulinella which enslaved a cyanobacterium independently of the ancestral plant). Endomyxa include almost all the free-living reticulose amoebae and most of the cercozoan parasites, specifically including the plasmodial (multinucleate) parasites of higher plants (Plasmodiophorida) and of invertebrates (Ascetosporea). The name Endomyxa (‘internal slime’) refers to their intracellular parasitic mode of feeding and associated plasmodial body form. However, within Endomyxa there are some amoebae (e.g. Arachnula) that have both filopodia and reticulopodia, and within Filosa an amoeboflagellate (still unnamed but belonging to Novel Clade 2) has recently been found to have both types of pseudopods, as do just a few cercomonads. Future discoveries might further undermine this distinction, but at present one can think of Filosa as being ancestrally gliding amoeboflagellates (similar to cercomonads) and Endomyxa as ancestrally free-living reticulose amoebae (similar to Gromia, but naked) with only a non-feeding flagellate dispersal phase.
Within Endomyxa, the marine parasites of invertebrates (Ascetosporea) seem to be more closely related to the reticulose marine Gromia and Filoreta. Unsurprisingly, the land-plant parasites are closer to the predominantly terrestrial aconchulinid reticulose amoebae such as Arachnula and Platyreta that tend to eat fungi or algae. Thus parasitism evolved independently in the plant and animal parasites and their plasmodial body form is a convergent adaptation to parasitism; plasmodiophorids retained zoospores for dispersal; Ascetosporea did not.
Within Filosa there are similarly interesting phylogenetic patterns and marine-freshwater dichotomies, some major lineages (e.g. chlorarachneans) being exclusively marine, and others exclusively soil/freshwater (e.g. cercomonads and glissomonads, two groups of zooflagellate gliders). The basal branching order of Filosa is poorly resolved, suggesting very rapid radiation of the major lineages. Four early diverging early-diverging lineages are the zooflagellates that glide on both cilia, the chlorarachnean algae, the marine metromonad gliding flagellates (possibly including the metopiids), and the Granofilosea (naked filose amoebae with granular pseudopods). They all appear to have mutually diverged before cercomonads evolved. The other testate filose amoebae (euglyphids, tectofilosids) are phylogenetically interspersed amongst the large cluster of zooflagellates that has long ciliary transitional regions and dense upper plates (Thaumatomonadida, Spongomonadida, Cryomonadida, Glissomonadida, Pansomonadida). There are weak indications that Glissomonadida are sisters to Pansomonadida and that sainouroids (zooflagellates with exceptionally short centrioles and transition regions and vestigial anterior cilia) may be related to one or both of them and that Thaumatomonadida and Spongomonadida may be sisters, but multigene evidence is essential to resolve the branching order within the long-transition region cluster and to confirm that they are a clade that may be sister to cercomonads.
Relationships of Cercozoa to Other Eukaryotes
The closest relatives of Cercozoa are the Retaria (Foraminifera and Radiozoa; Cavalier-Smith 1999). Radiozoa are all marine unicellular organisms with microtubule-supported axopodia; they comprise the classical polycystine Radiolaria (with silica skeletons), the Acantharea (with strontium sulphate skeletons), which both also possess axopodia, and the curious floating and axopodially swimming Sticholonche which is not mineralized. Radiozoa now excludes the Phaeodarea, which despite their silica skeleton and axopodia, clearly belong in Cercozoa. Phaeodarea appear to be related to the filosan group known as Thecofilosea, which includes filose testate amoebae without silica scales and also the exclusively marine ebriid flagellates, which like the Phaeodarea have a hollow silica endoskeleton, which might therefore have been a common ancestral character for both groups. A hollow silica endoskeleton is unknown in any other eukaryotes. Two groups of Cercozoa (the euglyphid testate amoebae and the amoeboflagellate thaumatomonads) bear silica scales on their cell surface and are relatively closely related, but apparently not sisters. They have been grouped together as the Imbricatea.
Almost all Foraminifera are also marine. The best rRNA sequence trees show Retaria as monophyletic and as sisters to Cercozoa. However this conclusion remains controversial because some single-gene trees separate Foraminifera from Radiozoa and place them within the cercozoan Endomyxa. The fact that Foraminifera share a single amino acid insertion within polyubiquitin with Cercozoa can also be used to argue that Foraminifera and Cercozoa may be closer to each other than either is to Radiozoa (Bass et al 2005). However, a shared deletion in 18S rRNA suggests that Cercozoa are monophyletic and exclude Foraminifera. Multigene trees should soon resolve whether Foraminifera are closer to Cercozoa or to Radiozoa.
Irrespective of the uncertainty over the precise position of Foraminifera, and thus the monophyly of Retaria and Cercozoa, the grouping of Retaria and Cercozoa as the supergroup Rhizaria is extremely robust. This makes it highly probable that the common ancestor of both Cercozoa and Retaria was a marine benthic amoeboflagellate protozoan with reticulose pseudopods. The name Rhizaria was chosen for this supergroup to perpetuate Dujardin’s idea enshrined in his name Rhizopoda, that slender root-like pseudopods were extremely important for the motility, feeding and general life style of a major group of eukaryotes (Cavalier-Smith 2002). Such tenuous root-like pseudopods can ramify around sediment particles and over surfaces to catch bacteria or eukaryotic prey over large areas or volumes with a minimal investment in protoplasm.
There are probably tens of thousands of rhizarian species with a net-like or thread-like body form which is the central lifestyle for the group which collectively constitutes the most abundant and speciose predators on earth in soils at the bottom of the oceans, lakes and rivers and in the plankton. This microscopic world of incredibly diverse thread or net like predators is ignored by textbooks but is ecologically far more important than lions, tigers, Tyrannosaurus, and wolves. Rhizarians have been the dominant predators for at least 550 million years. They appear to be most closely related to the chromalveolates. Recent multigene trees even suggest that they may have been derived from chromalveolate ancestors by the loss of photosynthesis.
References
Bass, D. & Cavalier-Smith, T. (2004) Phylum-specific environmental DNA analysis reveals remarkably high global biodiversity of Cercozoa (Protozoa). Int. J. Syst. Evol. Microbiol. 54, pp. 2393-2404.
Bass, D., Moreira, D., López-García, P., Polet, S., Chao, E. E., von der Heyden, S., Pawlowski, J. & Cavalier-Smith, T. (2005) Polyubiquitin insertions and the phylogeny of Cercozoa and Rhizaria. Protist 156, pp. 149-161.
Bass, D., Chao, E. E., Nikolaev, S., Yubuki, A., Ishida, K., Berney, C., Pakzad, U., Wylezich, U. & Cavalier-Smith, T. (2009) Phylogeny of novel naked filose and reticulose Cercozoa: Granofilosea cl. n. and Proteomyxidea revised. Protist 160, pp. 75-109.
Cavalier-Smith, T. (1997) Amoeboflagellates and mitochondrial cristae in eukaryotic evolution: megasystematics of the new protozoan subkingdoms Eozoa and Neozoa. Arch Protistenk 147, pp. 237-258.
Cavalier-Smith, T. (1998) A revised six-kingdom system of life. Biol Rev Camb Philos Soc 73, pp. 203-266.
Cavalier-Smith, T. (2002) The phagotrophic origin of eukaryotes and phylogenetic classification of Protozoa. Int. J. Syst. Evol. Microbiol. 52, pp. 297-354.
Cavalier-Smith, T. (1999) Principles of protein and lipid targeting in secondary symbiogenesis: euglenoid, dinoflagellate, and sporozoan plastid origins and the eukaryotic family tree. J. Euk. Microbiol. 46, pp. 347-366.
Cavalier-Smith, T. & Chao, E. E. (2003) Phylogeny and classification of phylum Cercozoa (Protozoa). Protist 154, pp. 341-358.
Cavalier-Smith, T., Lewis, R., Chao, E. E., Oates, B. & Bass, D. (2008) Morphology and phylogeny of Sainouron acronematica sp. n. and the ultrastructural unity of Cercozoa. Protist 159, pp. 591-620.
Title Illustrations

| Scientific Name | Eocercomonas ramosa |
|---|---|
| Creator | Photo: David Bass |
| Specimen Condition | Live Specimen |
| Collector | Alexandre Mylnikov |
| Copyright |
© 2008
David Bass

|
| Scientific Name | Filoreta marina |
|---|---|
| Location | Kartesh, White Sea, Russia |
| Reference | This image was published in Protist 160, David Bass, Ema E.-Y. Chao, Sergey Nikolaev, Akinori Yabuki, Ken-ichiro Ishida, Cédric Berney, Ursula Pakzad, Claudia Wylezich and Thomas Cavalier-Smith, Phylogeny of Novel Naked Filose and Reticulose Cercozoa: Granofilosea cl. n. and Proteomyxidea Revised, pp 75-109, Copyright Elsevier (2009). |
| Creator | Photo: David Bass |
| Specimen Condition | Live Specimen |
| Size | cell bodies c. 5-50 um; plasmodia cms in extent |
| Collector | Alexandre Mylnikov |
| Copyright | © 2009 Elsevier |
| Scientific Name | Euglypha cf ciliata |
|---|---|
| Reference | This image was published in Protist 158, E. Lara, T.J. Heger, E.A.D. Mitchell, R. Meisterfeld and F. Ekelund, SSU rRNA reveals a sequential increase in shell complexity among the euglyphid testate amoebae (Rhizaria: Euglyphida), pp. 229–237, Copyright Elsevier (2007). |
| Specimen Condition | Dead Specimen |
| Copyright | © 2007 Elsevier |
| Scientific Name | Lotharella amoeboformis |
|---|---|
| Comments | Amoeboid cells of Lotharella amoeboformis extending many filose pseudopodia |
| Copyright |
© 2001
Ken-ichiro Ishida

|
About This Page
This page is being developed as part of the Tree of Life Web Project Protist Diversity Workshop, co-sponsored by the Canadian Institute for Advanced Research (CIFAR) program in Integrated Microbial Biodiversity and the Tula Foundation.
David Bass

Oxford University, Oxford, United Kingdom
Tom Cavalier-Smith

Oxford University, Oxford, United Kingdom
Correspondence regarding this page should be directed to David Bass at
david.bass@zoo.ox.ac.uk
and Tom Cavalier-Smith at
tom.cavalier-smith@zoo.ox.ac.uk
Page copyright © 2009 David Bass and Tom Cavalier-Smith
 Page: Tree of Life
Cercozoa.
Authored by
David Bass and Tom Cavalier-Smith.
The TEXT of this page is licensed under the
Creative Commons Attribution-NonCommercial License - Version 3.0. Note that images and other media
featured on this page are each governed by their own license, and they may or may not be available
for reuse. Click on an image or a media link to access the media data window, which provides the
relevant licensing information. For the general terms and conditions of ToL material reuse and
redistribution, please see the Tree of Life Copyright
Policies.
Page: Tree of Life
Cercozoa.
Authored by
David Bass and Tom Cavalier-Smith.
The TEXT of this page is licensed under the
Creative Commons Attribution-NonCommercial License - Version 3.0. Note that images and other media
featured on this page are each governed by their own license, and they may or may not be available
for reuse. Click on an image or a media link to access the media data window, which provides the
relevant licensing information. For the general terms and conditions of ToL material reuse and
redistribution, please see the Tree of Life Copyright
Policies.
- First online 22 March 2009
- Content changed 22 March 2009
Citing this page:
Bass, David and Tom Cavalier-Smith. 2009. Cercozoa. Version 22 March 2009 (under construction). http://tolweb.org/Cercozoa/121187/2009.03.22 in The Tree of Life Web Project, http://tolweb.org/







 Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site
Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site