Alveolates
Alveolata
Brian S. Leander

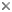
This tree diagram shows the relationships between several groups of organisms.
The root of the current tree connects the organisms featured in this tree to their containing group and the rest of the Tree of Life. The basal branching point in the tree represents the ancestor of the other groups in the tree. This ancestor diversified over time into several descendent subgroups, which are represented as internal nodes and terminal taxa to the right.

You can click on the root to travel down the Tree of Life all the way to the root of all Life, and you can click on the names of descendent subgroups to travel up the Tree of Life all the way to individual species.
For more information on ToL tree formatting, please see Interpreting the Tree or Classification. To learn more about phylogenetic trees, please visit our Phylogenetic Biology pages.
close boxIntroduction
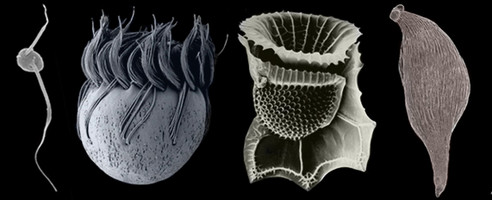
The Alveolata is a monophyletic group of primarily single-celled eukaryotes that have adopted extremely diverse modes of nutrition, such as predation, photoautotrophy and intracellular parasitism. Most alveolates fall into one of three main subgroups: ciliates (see the second cell, starting from the left, in the title image), dinoflagellates (see the third cell, starting from the left, in the title image), and apicomplexans (see the fourth cell, starting from the left, in the title image). Apicomplexans are obligate parasites; the name of the group stems from a novel apparatus at the anterior end of the parasites that facilitates host cell attachment and invasion. This “apical complex” consists of a tubulin-based (closed) “conoid” that serves as a spring-like scaffolding for extrusive organelles called “rhoptries”. Apicomplexans infect (mostly) animal cells, ranging from the epithelial cells in the intestines of insects and marine invertebrates to the red blood cells of primates, including humans (e.g. Plasmodium is the causative agent of malaria). Some dinoflagellates are also parasites, but most are either free-living predators or photoautotrophs in aquatic habitats (Taylor 1987). Many photosynthetic dinoflagellates are also consumers of bacteria and other microeukaryotes, a mode of nutrition called “mixotrophy” (Stoecker 1999). The photosynthetic and mixotrophic species are very important players in oceanic carbon cycles, and some cause harmful (toxic) algal blooms when cell densities reach exceedingly high levels (Taylor 1987). Dinoflagellates have novel cytoskeletal and nuclear features (e.g. permanently condensed chromosomes) that make them very distinctive among eukaryotes (Fensome et al. 1999). Ciliates are mainly dynamic predators that perform essential roles as consumers in microbial food webs, and like dinoflagellates and apicomplexans, this group is extremely diverse. Some ciliates, for instance, inhabit ruminant intestinal tracts, while others invade the flesh of fish. The best synapomorphies for ciliates are two heteromorphic nuclei and a cell cortex containing many cilia (i.e. short flagella) arranged in specific configurations.
Several known lineages of alveolates do not fit neatly within any of the three major subgroups discussed above, such as Colpodella (see the first and last cells in the title image), Chromera, Colponema, Ellobiopsids, Oxyrrhis, Rastrimonas, Parvilucifera, and Perkinsus (Brugerolle 2002, 2003; Dodge and Crawford 1971a,b; Fernandez et al. 1999; Mignot and Brugerolle 1975; Moore et al. 2008; Myl'nikov 1991, 2000; Noren et al. 1999; Perkins 1976, 1996; Saldarriaga et al. 2003; Siddall et al. 1997, 2001; Silberman 2004; Simpson and Patterson 1996). Most of these lineages blur the distinction between predator and parasite and possess combinations of features that provide compelling insights into the earliest stages of alveolate evolution (e.g. the mosaic of character states in distant ancestors) (Leander and Keeling 2003; Cavalier-Smith and Chao 2004; Siddall et al. 2001). Colpodellids and perkinsids, for instance, have biflagellated predatory stages in their lifecycles that are remarkably similar to one another (e.g. they possess an apical complex with an open-sided conoid) (Myl'nikov 1991, 2000; Simpson and Patterson 1996). Colpodellids form the nearest sister lineage to apicomplexans, and perkinsids form the nearest sister lineage to dinoflagellates; therefore, the features shared by colpodellids and perkinsids are inferred to have been retained from the most recent ancestor of apicomplexans and dinoflagellates through morphostasis (Kuvardina et al. 2002; Leander and Keeling, 2003, 2004; Leander et al. 2003; Saldarriaga et al. 2003; Moore et al. 2008). The clade consisting of this particular ancestor and all of its descendent is called the “Myzozoa” (Cavalier-Smith and Chao 2004).
The Alveolata forms a sister group to two major clades of photosynthetic eukaryotes, namely the (ochrophyte) stramenopiles and the clade consisting of haptophytes and cryptophytes. Alveolates contain both photosynthetic lineages, such as Chromera and many dinoflagellates, and non-photosynthetic lineages, such as ciliates, colpodellids, apicomplexans and perkinsids. Some members of the last two lineages have been shown to possess remnant (non-photosynthetic) plastids that are surrounded by four membranes, usually referred to as “apicoplasts” (Kilejian 1975; Matsuzaki et al. 2008; McFadden and Waller 1997; McFadden et al. 1996; Teles-Grilo et al. 2007; Wilson 1996). On one hand, dinoflagellates are known to have endosymbiotically gained and lost photosynthesis from different prey organisms several times independently throughout their history (Saldarriaga, J.F. et al. 2001; Shalchian-Tabrizi et al. 2006). On the other hand, there is no compelling cellular evidence that ciliates have ever had photosynthetic ancestors, despite the fact that many different lineages of ciliates are known to (temporarily) harbor photosynthetic symbionts (Johnson et al. 2007). This complex set of phylogenetic and cell biological circumstances has made inferences about the evolutionary origins of photosynthesis in alveolates a very challenging, contentious, and exciting area of biodiversity research.
Characteristics
Despite the large morphological differences between ciliates, apicomplexans and dinoflagellates, alveolates share several morphological features:
- A system of abutting membranous sacs, called “alveoli”, positioned beneath the plasma membrane (synapomorphy); the alveoli can be empty (e.g. colpodellids and apicomplexans) or filled with cellulosic material (e.g. thecate dinoflagellates and some ciliates)
- Distinct micropores through the cell surface that function in pinocytosis (synapomorphy)
- Similar extrusive organelles (e.g. trichocysts)
- Closed mitosis
- Tubular mitochondrial cristae
Discussion of Phylogenetic Relationships
Molecular phylogenetic analyses of several different protein genes have firmly demonstrated that apicomplexans and dinoflagellates are more closely related to one another than either is to ciliates, forming the Myzozoa (Fast et al. 2002; Leander and Keeling 2004; Gajadhar et al. 1991). Similar analyses have shown that perkinsids, which are parasites of mollusks and microeukaryotes, form the nearest sister lineage to dinoflagellates (Bushek et al. 2002; Ellis et al. 1998; Goggin and Barker 1993; Noren et al. 1999; Reece et al. 1997; Saldarriaga et al. 2003; Leander and Keeling 2004). Phylogenetic analyses of small subunit rRNA sequences indicate that free-living colpodellids and the photosynthetic coral symbiont Chromera are not specifically related to perkinsids, but instead are sister groups to apicomplexans (Kuvardina et al. 2002; Leander et al. 2003; Cavalier-Smith and Chao 2004; Moore et al. 2008). Phylogenies based on small subunit rDNA sequences have also indicated that ellobiopsids, enigmatic marine parasites of planktonic crustaceans, are alveolates with an uncertain position within the group (Silberman et al. 2004). Some morphological features of ellobiopsids indicate a close affinity with some parasitic relatives of dinoflagellates, such as “syndinians” (see the Dinoflagellates page). Overall, current evidence suggests that the early evolutionary history of alveolates involved voracious (colpodellid-like) predators of eukaryotic cells that ultimately diversified into (1) the specialized parasites seen in apicomplexans, perkinsids and ellobioposids; (2) the highly sophisticated consumers seen in ciliates; and (3) the complex assortment of predatory and (secondary) photoautotrophic cells seen in dinoflagellates.
References
Brugerolle, G. 2002. Cryptophagus subtilis: a new parasite of cryptophytes affiliated with the Perkinsozoa lineage. Europ. J. Protistol. 37:379-390.
Brugerolle, G. 2003. Apicomplexan parasite Cryptophagus renamed Rastrimonas gen. nov. Europ. J. Protistol., 39:101.
Bushek, D. et al. 2002. Serological affinities of the oyster pathogen Perkinsus marinus (Apicomplexa) with some dinoflagellates (Dinophyceae). J. Eukaryot. Microbiol. 49:11-16.
Cavalier-Smith, T. and Chao, E.E. 2004. Protalveolate phylogeny and systematics and the origins of Sporozoa and dinoflagellates (phylum Myzozoa nom. nov.). Europ. J. Protistol. 40:185-212.
Dodge, J.D. & Crawford, R.M. 1971a. Fine structure of the dinoflagellate Oxyrrhis marina: I. The general structure of the cell. Protistologica, 7:295-304.
Dodge, J.D. & Crawford, R.M. 1971b.Fine structure of the dinoflagellate Oxyrrhis marina: II. The flagellar system. Protistologica, 7:399-409.
Ellis, J.T., Morrison, D. A. & Jeffries, A.C. 1998. The phylum Apicomplexa: an update on the molecular phylogeny. In: Coombs, G., Vickerman, K., Sleigh, M. & Warren, A. (ed.), Evolutionary Relationships Among Protozoa. Kluwer Academic Publishers, Boston. p. 255-274.
Fast, N.M. et al. 2002. Re-examining alveolate evolution using multiple protein molecular phylogenies. J. Eukaryot. Microbiol. 49:30-37.
Fensome, R.A. et al. 1999. Dinoflagellate phylogeny revisited: reconciling morphological and molecular based phylogenies. Grana 38:66-80.
Fernández, I., Pardos, F., Benito, J. and Arroyo, N.L. 1999. Acrocoelus glossobalani gen. nov. et sp. nov., a protistan flagellate from the gut of the enteropneust Glossabalanus minutus. Europ. J. Protistol. 35:55-65.
Gajadhar, A.A., Marquardt, W.C., Hall, R., Gunderson, J., Carmona, E.V.A. and Sogin, M.L. 1991. Ribosomal RNA sequences of Sarcocystis muris, Theileria annulata, and Crypthecodinium cohnii.reveal evolutionary relationships among apicomplexans, dinoflagellates, and ciliates. Mol. Biochem. Parasit. 45:147-154.
Goggin, C.L. & Barker, S.C. 1993. Phylogenetic position of the genus Perkinsus (Protista, Apicomplexa) based on small subunit ribosomal RNA. Mol. Biochem. Parasitol., 60:65-70.
Harper, J. T., Waanders, E. and Keeling, P. J. 2005. On the monophyly of chromalveolates using a six-protein phylogeny of eukaryotes. Int. J. System. Evol. Microbiol. 55:487-496.
Kilejian, A. 1975. Circular mitochondrial DNA from the avian malarial parasite Plasmodium lophurae. Biochim. Biophys. Acta 390:267-284.
Kuvardina, O.N., Leander, B.S., Aleshin, V.V., Mylnikov, A. P., Keeling, P.J. & Simdyanov, T. G. 2002. The phylogeny of colpodellids (Eukaryota, Alveolata) using small subunit rRNA genes suggests they are the free-living ancestors of apicomplexans. J. Eukaryot. Microbiol., 49:498-504.
Johnson, M.D., Oldach, D., Delwiche, C.F., and Stoecker, D.K. 2007. Retention of transcriptionally active cryptophyte nuclei by the ciliate Myrionecta rubra. Nature. 445:426-428.
Leander, B. S. and P. J. Keeling. 2003. Morphostasis in alveolate evolution. Trends in Ecology and Evolution 18(8):395-402.
Leander, B. S. and P. J. Keeling. 2004. Early evolutionary history of dinoflagellates and apicomplexans (Alveolata) inferred from HSP90 and actin phylogenies. J. Phycol. 40:341-250.
Leander, B.S., Kuvardina, O.N., Aleshin, V.V., Mylnikov, A.P. and Keeling, P.J. 2003. Molecular phylogeny and surface morphology of Colpodella edax (Alveolata): Insights into the phagotrophic ancestry of apicomplexans. J. Eukaryot. Microbiol., 50:334-340.
Matsuzaki, M., Kuroiwa, H., Kuroiwa, T., et al. 2008, A cryptic algal group unveiled: A plastid biosynthesis pathway in the oyster parasite Perkinsus marinus. Mol. Biol. Evol. 25:1167-1179.
McFadden, G.I. and R.F. Waller.1997: Plastids in parasites of humans. Bioessays 19:1033-1040.
McFadden, G.I. et al. 1996. Plastid in human parasites. Nature 381:482.
Mignot, J. P. and Brugerolle, G. 1975. Étude ultrastructurale du flagellé phagotrophe Colponema loxodes Stein. Protistologica, 11:429-444.
Moore, R.B., Obornik, M., Janouskovec, J., Chrudimsky, T., Vancova, M., Green, D.H., Wright, S.W., Davies, N.W., Bolch, C.J.S., Heimann, K., Slapeta, J., Hoegh-Guldberg, O., Logsdon, J.M. and Carter, D.A. (2008) A photosynthetic alveolate closely related to apicomplexan parasites. Nature. 451:959-963.
Mylnikov, A. P. 1991. The ultrastructure and biology of some representatives of order Spiromonadida (Protozoa). Zoologichesky Zhurnal, 70:5-15.
Mylnikov, A. P. 2000. The new marine carnivorous flagellate Colpodella pontica (Colpodellida, Protozoa). Zoologichesky Zhurnal, 79:261-266.
Norén, F., Moestrup, O. and Rehnstam-Holm, A.-S. 1999. Parvilucifera infectans Norén et Moestrup gen. et sp. nov. (Perkinsozoa phylum nov.): a parasitic flagellate capable of killing toxic microalgae. Europ. J. Protistol., 35:233-254.
Perkins, F. O. 1976. Zoospores of the oyster pathogen, Dermocystidium marinum. I. Fine structure of the conoid and other sporozoan-like organelles. J. Parasitol., 62:959-974.
Perkins, F. O. 1996. The structure of Perkinsus marinus (Mackin, Owen and Collier, 1950) Levine, 1978 with comments on the taxonomy and phylogeny of Perkinsus spp. J. Shell. Res., 15:67-87.
Reece, K.S. et al. 1997. Phylogenetic analysis of Perkinsus based on actin gene sequences. J. Parasitol. 83:417-423.
Saldarriaga, J.F. et al. (2001) Dinoflagellate nuclear SSU rDNA phylogeny suggests multiple plastid losses and replacements. J. Mol. Evol. 53, 204-213.
Saldarriaga, J. F., McEwan, M. L., Fast, N. M., Talyor, F. J. R. and Keeling, P. J. 2003. Multiple protein phylogenies show that Oxyrrhis marina and Perkinsus marinus are early branches of the dinoflagellate lineage. Int. J. Syst. Evol. Microbiol.: 53:355-365.
Shalchian-Tabrizi, K., Minge, M.A., Cavalier-Smith, T., Nedreklepp, J.M., Klaveness, D., and Jakobsen, K.S. 2006. Combined heat shock protein 90 and ribosomal RNA sequence phylogeny supports multiple replacements of dinoflagellate plastids. J. Eukaryot. Microbiol. 53:217-224.
Siddall, M. E., Reece, K. S., Graves, J. E. and Burreson, E. M. 1997. “Total evidence” refutes the inclusion of Perkinsus species in the phylum Apicomplexa. Parasitology, 115:165-176.
Siddall, M. E., Reece, K. S., Nerad, T. A. and Burreson, E. M. 2001. Molecular determination of the phylogenetic position of a species in the genus Colpodella. Am. Mus. Nov., 3314:1-10.
Silberman, J.D., Collins, A.G., Gershwin, L.A., Johnson, P.J. and Roger, A.J. 2004. Ellobiopsids of the genus Thalassomyces are alveolates. J. Eukaryot. Microbiol. 51:246-252.
Simpson, A. G. B. and Patterson, D. J. 1996. Ultrastructure and identification of the predatory flagellate Colpodella pugnax Cienkowski (Apicomplexa) with a description of Colpodella turpis n. sp. and a review of the genus. Syst. Parasitol., 33:187-198.
Stoecker, D.K. (1999) Mixotrophy among dinoflagellates. J. Eukaryot. Microbiol. 46:397-401.
Taylor, F.J.R. (1987) The Biology of Dinoflagellates, Blackwell Scientific Publications.
Teles-Grilo, M.L., Tato-Costa, J., Duarte, S.M., et al. 2007. Is there a plastid in Perkinsus atlanticus (Phylum Perkinsozoa)? Europ. J. Protistol. 43:163-167.
Wilson, R.J. et al. 1996. Complete gene map of the plastid-like DNA of the malaria parasite Plasmodium falciparum. J. Mol. Biol. 261:155-172.
Information on the Internet
- Introduction to the Alveolates. UCMP Berkeley.
Title Illustrations

| Scientific Name | From left to right: Colpodella, Halteria (Ciliate), Ornithocercus (Dinoflagellate), Monocystis (Apicomplexan) |
|---|---|
| Specimen Condition | Scanning Electron Micrographs |
| Copyright |
© Brian S. Leander

|
About This Page
This page is being developed as part of the Tree of Life Web Project Protist Diversity Workshop, co-sponsored by the Canadian Institute for Advanced Research (CIFAR) program in Integrated Microbial Biodiversity and the Tula Foundation.
Brian S. Leander

The University of British Columbia, Vancouver, British Columbia, Canada
Correspondence regarding this page should be directed to Brian S. Leander at
bleander@interchange.ubc.ca
Page copyright © 2009 Brian S. Leander
 Page: Tree of Life
Alveolates. Alveolata.
Authored by
Brian S. Leander.
The TEXT of this page is licensed under the
Creative Commons Attribution-NonCommercial License - Version 3.0. Note that images and other media
featured on this page are each governed by their own license, and they may or may not be available
for reuse. Click on an image or a media link to access the media data window, which provides the
relevant licensing information. For the general terms and conditions of ToL material reuse and
redistribution, please see the Tree of Life Copyright
Policies.
Page: Tree of Life
Alveolates. Alveolata.
Authored by
Brian S. Leander.
The TEXT of this page is licensed under the
Creative Commons Attribution-NonCommercial License - Version 3.0. Note that images and other media
featured on this page are each governed by their own license, and they may or may not be available
for reuse. Click on an image or a media link to access the media data window, which provides the
relevant licensing information. For the general terms and conditions of ToL material reuse and
redistribution, please see the Tree of Life Copyright
Policies.
- Content changed 16 September 2008
Citing this page:
Leander, Brian S. 2008. Alveolates. Alveolata. Version 16 September 2008 (under construction). http://tolweb.org/Alveolates/2379/2008.09.16 in The Tree of Life Web Project, http://tolweb.org/




 Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site
Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site