Multicotyle purvisi and Lobatostoma manteri
are discussed as examples (Rohde, 1972, 1973, further references therein).
Pre-adult juveniles differ from adults in that only the latter produce
sperm and egg cells. An oral sucker is lacking; the mouth opening is
located anteriorly to the pharynx which opens into the single caecum.
Two testes are found in the middle of the body and anteriorly to them
an ovary. The vitellarium extends along both sides of the body and is
confluent posteriorly. The uterus occupies much of the anterior half
of the body. It opens, together with the sperm duct, close to the anterior
end of the body. A septate oviduct is characteristic of all aspidogastreans
which have been examined in this respect (Fig.1).

Click on an image to view larger version & data in a new window

Figure 1. The female reproductive organs near the ovary
of Multicotyle purvisi.
Note the septate oviduct, indicated by the narrow lumen surrounded
by a thick wall in the cross-section (redrawn from Rohde, 1972).
In both M. purvisi and L. manteri,
a Laurer's canal extends from the female reproductive ducts to the dorsal
surface where it opens to the outside (Fig.1 and Fig. 2).

Click on an image to view larger version & data in a new window
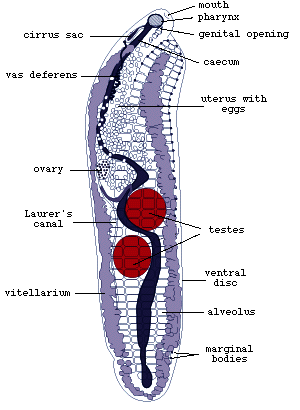
Figure 2. Adult Multicotyle
purvisi (redrawn from Rohde, 1972).
L. manteri has a powerfully developed eversible copulatory
organ, a cirrus. In M. purvisi, the ventral disc extends
along most of the body (Fig.2). In its anterior half, it is separated
from the main body by a septum of connective tissue (Fig.3).

Click on an image to view larger version & data in a new window
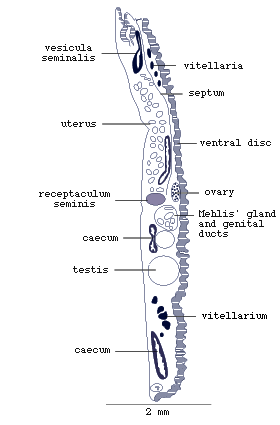
Figure 3. Sagittal (longitudinal section along the midline)
of adult Multicotyle purvisi.
Note the septum of connective tissue between the anterior ventral
and dorsal parts of the body (redrawn from Rohde, 1972).
It is subdivided into four longitudinal rows of alveoli (suckerlets).
So-called marginal bodies are located between the marginal alveoli.
Electron-microscopic studies of the marginal bodies of M. purvisi
and L. manteri have shown that they are ampullae (Fig.4),
which serve to store secretion produced by the marginal gland cells
in the dorsal parts of the marginal alveoli. The secretion is transported
in the ducts to the ampullae.

Click on an image to view larger version & data in a new window
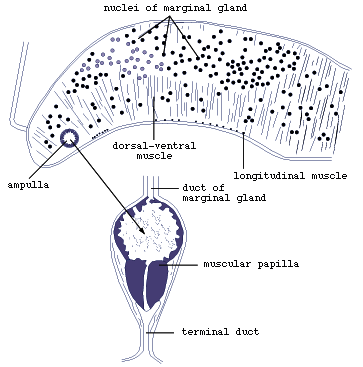
Figure 4. Marginal organ of Lobatostoma
manteri (redrawn from Rohde, 1973).
The ampullae open on the ventral surface through short ducts (Figs.4
and 5).

Click on an image to view larger version & data in a new window
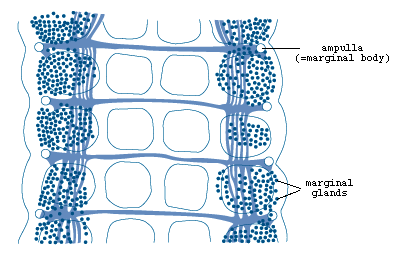
Figure 5. Marginal organs of Multicotyle
purvisi showing marginal glands, ampullae
and connecting ducts (redrawn from Rohde, 1972).
Nervous system
The nervous system both of the larva and the adult is of extraordinary
complexity (Figs. 6 and 7).

Click on an image to view larger version & data in a new window
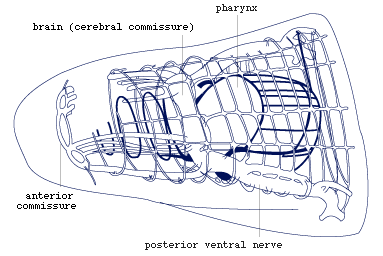
Figure 6. Nervous system in anterior part of body. Note
that the brain is the dorsal part of a ring commissure, and that there
is a second more external ring commissure at the same level (redrawn
from Rohde, 1972).

Click on an image to view larger version & data in a new window
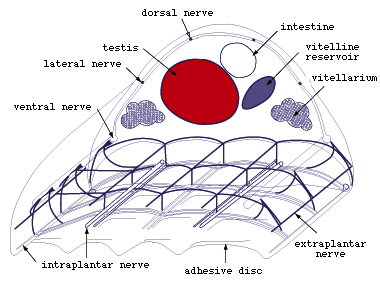
Figure 7. Main nerves in middle part of Multicotyle
purvisi (redrawn from Rohde, 1972).
Whereas most flatworms have a ladder system of nerves consisting of
a number of longitudinal nerve cords (connectives) below the surface
epidermis or tegument connected by ring commissures, both the larva
and the adult of Multicotyle purvisi have two such
systems in the anterior part of the body, one below the surface and
one surrounding the mouth cavity. Furthermore, the number of connectives
is very large, i.e., 14, and the brain is the dorsal part of a ring
commissure surrounding the mouth cavity (Fig.6). The latter observation
may have important phylogenetic implications, it may indicate that the
brain in the Platyhelminthes and perhaps in all invertebrates has evolved
as a processing centre in the interior of the body and not, as usually
assumed, as an aggregation of nerve cells around anterior sensory receptors.
- Among the posterior nerves, the ventral connectives are best developed
(Fig.7). Some branches of these connectives enter the ventral disc of
the adult or posterior sucker of the larva, and other branches enter
the pharynx of the adult and larva. Intestine and septum dorsal to the
ventral disc are also well innervated. Anteriorly and posteriorly, the
longitudinal nerves are connected by well developed commissures. Of
particular importance, parts of posterior nerves were found to be surrounded
by a multilamellate sheath, one of the few instances of glial elements
in the nervous system of flatworms. Clusters of glandular, apparently
neurosecretory cells were shown to occur at the junctions of connectives
and commissures dorsal and lateral to the ventral disc.
Sensory receptors
Electron microscopic studies of Lobatostoma manteri
and Multicotyle purvisi have shown an amazing variety
of sensory receptors, which differ in the absence or presence of cilia,
in the shape and length of the cilia, in the absence or presence of
ciliary rootlets and their shape, in the number of axonemal microtubules,
and whether they are part of a complex organ or not. In pre-adults of
the first species, eight (and possibly a further six) types, in adults
of the second species seven (and possibly a further two) types were
found (Fig.8) (Rohde, 1989a, 1990; Rohde and Watson, 1989a).
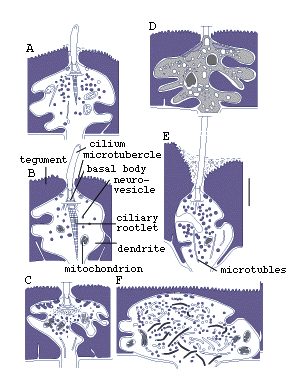
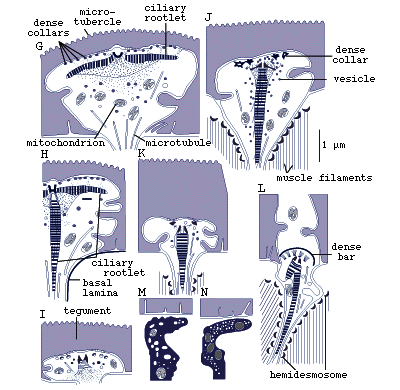
Figure 8. Sensory receptors of pre-adult Lobatostoma
manteri. Note differences in presence
or absence of a cilium or a ciliary rootlet, length of cilium, shape
of ciliary rootlet, presence or absence of tubules, location in the
tegument (redrawn from Rohde, 1989a).
It has been estimated that pre-adult L. manteri has
at least 8500 surface receptors and many sub-surface receptors as well.
References
Bailey, H. H. and Tompkin, S. S. (1971). Ultrastructure of the integument of Aspidogaster conchicola. Journal of Parasitology 57, 848-854.
Bakker, K. E. and Diegenbach, P. C. (1973). The ultrastructure of spermatozoa of Aspidogaster conchicola Baer, 1826 (Aspidogastridae, Trematoda). Netherlands Journal of Zoology 23, 345-346.
Bakker, K. E. and Diegenbach, P. C. (1974). The structure of the opisthaptor of Aspidogaster conchicola Baer, 1826 (Aspidogastridae, Trematoda). Netherlands Journal of Zoology 24, 162-170.
Fredericksen, D. W. (1972). Morphology and taxonomy of Cotylogaster occidentalis (Trematoda: Aspidogastrae). Journal of Parasitology 58, 1110-1116.
Gibson, D. I. and Chinabut, S. (1984). Rohdella siamensis gen. et sp. nov. (Aspidogastridae: Rohdellinae subfam. nov.) from freshwater fishes in Thailand, with a reorganization of the classification of the subclass Aspidogastrea. Parasitology 88, 383-393.
Halton, D. W. (1972). Ultrastructure of the alimentary tract of Aspidogaster conchicola (Trematoda: Aspidogastrea). Journal of Parasitology 58, 455-467.
Halton, D. W. and Lyness, R. A. W. (1971). Ultrastructure of the tegument and associated structures of Aspidogaster conchicola (Trematoda: Aspidogastrea). Journal of Parasitology 57, 1198-1210.
Hathaway, R. P. (1972a). Some observations on the ultrastructure of the female reproductive system of Aspidogaster conchicola (Trematoda: Aspidobothria). Journal of the Colorado-Wyoming Academy of Science 7, 114.
Hathaway, R. P. (1972b). The fine structure of the cecal epithelium of the trematode Aspidogaster conchicola von Baer, 1827. Proceedings of the Helminthological Society of Washington 39, 101-107.
Hathaway, R. P. (1974). The fine structure and development of spermatozoa of the trematode Aspidogaster conchicola von Baer, 1827. Journal of the Colorado-Wyoming Academy of Science 7, 70-71.
Hendrix, S. S. and Short, R. B. (1972). The juvenile of Lophotaspis interiora Ward and Hopkins, 1931 (Trematoda: Aspidobothria). Journal of Parasitology 58, 63-67.
Ip, H. S. and Desser, S. S. (1984). Transmission electron microscopy of the tegumentary sense organs of Cotylogaster occidentalis (Trematoda: Aspidogastrea). Journal of Parasitology 70, 563-575.
Ip, H. S., Desser, S. S. and Weller, I. (1982). Cotylogaster occidentalis (Trematoda: Aspidogastrea): scanning electron microscopic observations of sense organs and associated surface structures. Transactions of the American Microscopical Society 101, 253-261.
LoVerde, P. T. and Fredericksen, D. W. (1978). The chromosomes of Cotylogaster occidentalis and Cotylaspis insignis (Trematoda: Aspidogastrea) with evolutionary considerations. Proceedings of the Helminthological Society of Washington 45, 158-161.
Oliva, M. M. and Luque, J. L. (1989). The genus Lobatostoma (Trematoda: Aspidocotylea) in the Pacific coast of South America, with description of Lobatostoma veranoi new species, parasite of Menticirrhus ophicephalus (Teleostei: Sciaenidae). Memorias do Instituto Oswaldo Cruz, Rio de Janeiro 84, 167-170.
Ramulu, G. R., Rao, L. N. and Simha, S. S. (1980). Nervous system of Lissemysia indica Sinha, 1935: Aspidogasteridae. Indian Journal of Parasitology 4, 81-83.
Rohde, K. (1972). The Aspidogastrea, especially Multicotyle purvisi Dawes, 1941. Advances in Parasitology 10, 77-151.
Rohde, K. (1973). Structure and development of Lobatostoma manteri sp. nov. (Trematoda: Aspidogastrea) from the Great Barrier Reef, Australia. Parasitology 66, 63-83.
Rohde, K. (1982). The flame cells of a monogenean and an aspidogastrean, not composed of two interdigitating cells. Zoologischer Anzeiger 209, 311-314.
Rohde, K. (1989a). At least eight types of sense receptors in an endoparasitic flatworm: a counter-trend to sacculinization. Naturwissenschaften 76, 383-385.
Rohde, K. (1989b). Ultrastructure of the protonephridial system of Lobatostoma manteri (Trematoda, Aspidogastrea). Journal of Submicroscopic Cytology and Pathology 21, 599-610.
Rohde, K. (1990). Ultrastructure of the sense receptors of adult Multicotyle purvisi (Trematoda, Aspidogastrea). Zoologica Scripta 19, 233-241.
Rohde, K. (1994). The minor groups of parasitic Platyhelminthes. Advances in Parasitology 33, 145 - 234.
Rohde, K. and Watson, N. (1989a). Sense receptors in Lobatostoma manteri (Trematoda, Aspidogastrea). International Journal for Parasitology 19, 847-858.
Rohde, K. and Watson, N. (1989b). Ultrastructure of the marginal glands of Lobatostoma manteri (Trematoda, Aspidogastrea). Zoologischer Anzeiger 223, 301-310.
Rohde, K. and Watson, N. A. (1991). Vitellogenesis of Rugogaster hydrolagi (Trematoda, Aspidogastrea). Annales de Parasitologie Humaine et Comparée 66, 273-280.
Rohde, K. and Watson, N. A. (1992). Ultrastructure of tegument, ventral sucker and rugae of Rugogaster hydrolagi (Trematoda, Aspidogastrea). International Journal for Parasitology
Rohde, K., Watson, N. A. and Cribb, T. (1991). Ultrastructure of sperm and spermatogenesis of Lobatostoma manteri (Trematoda, Aspidogastrea). International Journal for Parasitology 21, 409-419.
Rohde, K., Heap, M., Hayward, C. J. and Graham, K. J. (1992). Calicotyle australiensis n. sp. and Calicotyle sp. (Monogenea, Monopisthocotylea) from the rectum and rectal glands, and Rugogaster hydrolagi Schell, 1973 (Trematoda, Aspidogastrea) from the rectal glands of holocephalans off the coast of southeastern Australia. Systematic Parasitology 21, 69-79.
Schell, S. C. (1973). Rugogaster hydrolagi gen. et sp. n. (Trematoda: Aspidobothrea: Rugogastridae fam. n.) from the ratfish, Hydrolagus colliei (Lay and Bennett, 1839). Journal of Parasitology 59, 803-805.
Thoney, D. A. and Burreson, E. M. (1987). Morphology and development of the adult and cotylocidium of Multicalyx cristata (Aspidocotylea), a gall bladder parasite of elasmobranchs. Proceedings of the Helminthological Society of Washington 54, 96-104.
Timofeeva, T. A. (1971). [The structure of the nervous system of Aspidogaster conchicola K.Baer, 1827 (Trematoda, Aspidogastrea)]. Parazitologiya 5, 517-523 (In Russian).
Timofeeva, T. A. (1972). [Glandular system of adhesive disc in Aspidogaster limacoides (Trematoda, Aspidogastrea)]. Zoologicheskij Zhurnal 51, 1071-1073 (In Russian).
Trimble III, J. L., Bailey, H. H. and Nelson, E. N. (1971). Aspidogaster conchicola (Trematoda: Aspidobothrea): histochemical localization of acid and alkaline phosphatases. Experimental Parasitology 29, 457-462.
Trimble III, J. J., Bailey, H. H. and Sheppard, A. (1972). Aspidogaster conchicola : histochemical localization of carboxylic ester hydrolases. Experimental Parasitology 32, 181-190.
Watson, N. A. and Rohde, K. (1991). Ultrastructure of spermatogenesis and sperm of Multicotyle purvisi (Trematoda, Aspidogastrea). Zoomorphology 110, 347-356.
Watson, N. A. and Rohde, K. (1992a). Ultrastructure of sperm and spermatogenesis of Rugogaster hydrolagi, Schell 1973 (Platyhelminthes, Trematoda, Aspidogastrea, Rugogastridae). Parasitology Research 78,516-524.
Watson, N. A. and Rohde, K. (1992b). Ultrastructure of the flame bulbs and protonephridial capillaries of Rugogaster hydrolagi (Platyhelminthes, Trematoda, Aspidogastrea). Annales de Parasitologie Humaine et Comparée 67, 67-74.
















 Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site
Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site