Pyrenulales
Cecile Gueidan- Monoblastaceae
- Pyrenulaceae
- Requienellaceae
Introduction
Pyrenulales includes mostly lichenized taxa, the majority of them belonging to Anisomeridium and Pyrenula, both with more than 100 accepted species. These lichenized Pyrenulales are associated with green algae belonging exclusively to the Trentepohliaceae, a family characterized by its orange carotenoid pigments. This order also includes some saprophytic non-lichenized taxa. The great majority of the lichenized Pyrenulales colonize trees, where they occur exclusively on bark. Only a few species such as those in the genus Acrocordia grow on rocks, mostly on limestone. The non-lichenized taxa are found on bark, leaves or wood. Some Pyrenulales occur in temperate climates, but they are predominantly tropical, where they are very diverse as epiphytes in rainforests. Although many recent collecting efforts have been carried out in the tropics (Aptroot 1997, 2002, 2003; Aptroot et al 1997; Aptroot and Seaward 1999; Aptroot and Sipman 1991), many regions are still understudied, and the species diversity of this order is probably highly underestimated (Aptroot 2001; Aptroot and Sipman 1997).
Characteristics
Lichenized Pyrenulales are characterized by a thin thallus, either immersed in the substrate or superficial. The structure of their thalli is never as complex as in some other lichens, and they never form foliose or fruticose thalli (Aptroot 1991). Vegetative propagules such as soredia and isidia, typically found in lichens from the Lecanoromycetes, are absent. Similarly, secondary metabolites, so informative for species identification within the Lecanoromycetes, are very rare in this order, limited to a few species such as Pyrenula nitida which contain anthraquinones (Harris 1989).
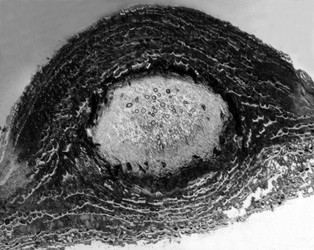
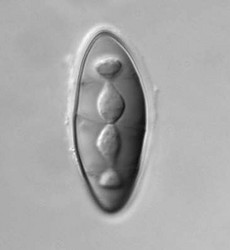
Pyrenulales are characterized by perithecial ascomata that are ostiolate, often papillate, and sometimes aggregated. The excipulum is mostly formed of a textura intritica, which may or may not be carbonized (Aptroot 1991). The hamathecium generally consists of narrow trabeculate pseudoparaphyses, subsequently replaced by unbranched paraphyses in the family Pyrenulaceae (Kirk et al. 2001). In the Requiellenaceae, the hamathecium differs by having unbranched and sparsely septate paraphyses (Boise, 1986). Asci are functionally bitunicate and they have ascohymenial origins (Janex-Favre 1971). Spores are colorless or brown, transversally septate to muriform.
Discussion of Phylogenetic Relationships
The recent contribution from molecular phylogenetic studies has changed past views on the taxonomic delimitation of the order Pyrenulales, first known as including the four families Monoblastiaceae, Pyrenulaceae, Trypetheliaceae, and Requienellaceae (see also Geiser et al. 2006). The family Trypetheliaceae was shown not to be related to the Pyrenulales, but nested with the Dothideomycetes (del Prado et al. 2006). This result, as well as previous morphological evidence (Boise 1986; Eriksson and Hawksworth 1992; Harris 1989), sheds light on the close phylogenetic relationship between the Pyrenulaceae and the two families Monoblastiaceae and Requienellaceae. Additional molecular investigations are necessary to further assess the circumscription of this order.
References
Aptroot A. 1991. A monograph of the Pyrenulaceae (excluding Anthracothecium and Pyrenula) and the Requienellaceae, with notes on the Pleomassariaceae, the Trypetheliaceae and Mycomicrothelia (lichenized and non-lichenized ascomycetes. 178 p.
Aptroot A. 1997. Additional lichen records from Australia, 30. New records of Pyrenocarps. Australasian Lichenology Newsletter 40: 4-7.
Aptroot A. 2001. Lichenized and saprobic fungal biodiversity of a single Elaeocarpus tree in Papua New Guinea, with the report of 200 species of ascomycetes associated with one tree. Fungal Diversity 6: 1-11.
Aptroot A. 2002. New and interesting lichens and lichenicolous fungi in Brazil. Fungal Diversity 9: 15-45.
Aptroot A. 2003. Pyrenocarpous lichens and related non-lichenized ascomycetes from Taiwan. Journal of the Hattori Botanicaly Laboratory 93: 155-173.
Aptroot A, Diederich P, Sérusiaux E, Sipman HJM. 1997. Lichens and lichenicolous fungi from New Guinea. 220 p.
Aptroot A, Seaward MRD. 1999. Annotated checklist of Hong Kong lichens. Tropical Bryology 17: 57-101.
Aptroot A, Sipman HJM. 1991. New lichens and lichen records for New Guinea. Willdenowia 20: 221-256.
Aptroot A, Sipman HJM. 1997. Diversity of lichenized fungi in the tropics, In: Hyde KD eds. Biodiversity of Tropical Microfungi. Hong Kong: Hong Kong University Press, p 93-106.
Boise J. 1986. Requienellaceae, a new family of Loculoascomycetes. Mycologia 78: 37-41.
Eriksson OE, Hawksworth DL. 1992. Notes on ascomycete systematics. Systema Ascomycetum 11: 49-82.
Geiser DM, Gueidan C, Miadlikowska J, Lutzoni F, Kauff F, Hofstetter V, Fraker E, Schoch C, Tibell L, Untereiner WA, Aptroot A. 2006. Eurotiomycetes: Eurotiomycetidae and Chaetothyriomycetidae. Mycologia 98: 1054-1065.
Harris RC. 1989. A sketch of the family Pyrenulaceae (Melanommatales) in eastern North America. Memoirs of the New York Botanical Garden 49: 74-107.
Hibbett, D. S., M. Binder, J. F. Bischoff, M. Blackwell, P. F. Cannon, O. E. Eriksson, S. Huhndorf, T. James, P. M. Kirk, R. Lücking, T. Lumbsch, F. Lutzoni, P. B. Matheny, D. J. Mclaughlin, M. J. Powell, S. Redhead, C. L. Schoch, J. W. Spatafora, J. A. Stalpers, R. Vilgalys, M. C. Aime, A. Aptroot, R. Bauer, D. Begerow, G. L. Benny, L. A. Castlebury, P. W. Crous, Y.-C. Dai, W. Gams, D. M. Geiser, G. W. Griffith, C. Gueidan, D. L. Hawksworth, G. Hestmark, K. Hosaka, R. A. Humber, K. Hyde, J. E. Ironside, U. Kõljalg, C. P. Kurtzman, K.-H. Larsson, R. Lichtwardt, J. Longcore, J. Miądlikowska, A. Miller, J.-M. Moncalvo, S. Mozley-Standridge, F. Oberwinkler, E. Parmasto, V. Reeb, J. D. Rogers, C. Roux, L. Ryvarden, J. P. Sampaio, A. Schüßler, J. Sugiyama, R. G. Thorn, L. Tibell, W. A. Untereiner, C. Walker, Z. Wang, A. Weir, M. Weiß, M. M. White, K. Winka, Y.-J. Yao, and N. Zhang. 2007. A higher-level phylogenetic classification of the Fungi. Mycological Research 111: 509-547.
Janex-Favre MC. 1971. Recherches sur l'ontogonie, l'organisation et les asques de quelques pyrenolichens. Rev. Bryol. & Lichenol. 37: 421-469.
Kirk PM, David JC, Stalpers JA. 2001. Ainsworth & Bisby's Dictionary of the Fungi. Wallingford, UK: CAB International. 650 p.
del Prado RI, Schmitt I, Kautz S, Palice R, Lücking R, Lumbsch HT. 2006. Morphological and molecular evidence place the Tryphetheliaceae in the Dothideomycetes. Mycological Research 110: 511-520.
Title Illustrations

| Scientific Name | Pyrenula pseudobufonia |
|---|---|
| Identified By | C. Gueidan |
| Body Part | perithecium |
| View | cross-section |
| Copyright |
©
Cecile Gueidan

|
| Scientific Name | Pyrenula pseudobufonia |
|---|---|
| Identified By | C. Gueidan |
| Life Cycle Stage | transversally septate spore |
| Copyright |
©
Cecile Gueidan

|
About This Page
Cecile Gueidan

Duke University, Durham, North Carolina, USA
Correspondence regarding this page should be directed to Cecile Gueidan at
cecile.gueidan@duke.edu
Page copyright © 2008 Cecile Gueidan
All Rights Reserved.
- First online 21 December 2007
- Content changed 29 January 2008
Citing this page:
Gueidan, Cecile. 2008. Pyrenulales. Version 29 January 2008 (under construction). http://tolweb.org/Pyrenulales/29306/2008.01.29 in The Tree of Life Web Project, http://tolweb.org/





 Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site
Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site