Echinodermata
Spiny-skinned animals: sea urchins, starfish, and their allies
Gregory A. Wray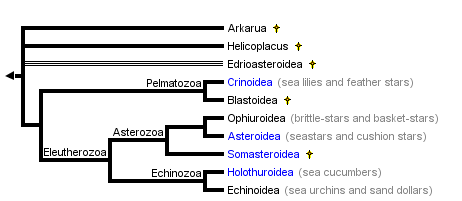

This tree diagram shows the relationships between several groups of organisms.
The root of the current tree connects the organisms featured in this tree to their containing group and the rest of the Tree of Life. The basal branching point in the tree represents the ancestor of the other groups in the tree. This ancestor diversified over time into several descendent subgroups, which are represented as internal nodes and terminal taxa to the right.

You can click on the root to travel down the Tree of Life all the way to the root of all Life, and you can click on the names of descendent subgroups to travel up the Tree of Life all the way to individual species.
For more information on ToL tree formatting, please see Interpreting the Tree or Classification. To learn more about phylogenetic trees, please visit our Phylogenetic Biology pages.
close boxSummary phylogenetic hypothesis of the Echinodermata, based on David and Mooi (1997), Littlewood et al. (1997), and Sumrall and Sprinkle (1997). Note that the phylogenetic position of most fossil echinoderms is still uncertain, and a number of additional extinct taxa will be added to this tree in the future.
Introduction
Echinoderms form a well-defined and highly-derived clade of metazoans. They have attracted much attention due to their extensive fossil record, ecological importance in the marine realm, intriguing adult morphology, unusual biomechanical properties, and experimentally manipulable embryos. The approximately 7,000 species of extant echinoderms fall into five well-defined clades: Crinoidea (sea lilies and feather stars), Ophiuroidea (basket stars and brittle stars), Asteroidea (starfishes), Echinoidea (sea urchins, sand dollars, and sea biscuits), and Holothuroidea (sea cucumbers). The phylogenetic position of the Concentricycloidea (sea daisies; 2 species), remains controversial (Baker et al. 1986; Smith 1988b; Pearse and Pearse 1994; Mooi et al. 1997).
Approximately 13,000 echinoderm species are known from the fossil record. All Mesozoic and Cenozoic forms clearly fall into the five extant clades, but the Paleozoic record contains numerous distinct and often bizarre forms that have been placed into approximately 15 additional classes. Phylogenetic relationships, and in some cases status as monophyletic groups, remains unclear for the extinct classes. Unquestionable echinoderms first appear in the fossil record during the mid-Cambrian. Arkarua, a possible echinoderm, has been described from the Vendian (latest Proterozoic) (Gehling 1987).
Characteristics
Synapomorphies of the Echinodermata
Echinoderms are among the most distinctive of all animal phyla. Inclusion in the phylum is readily diagnosable on basis of the four synapomorphies below. Most of these features are present, or can be inferred, even in the earliest fossils. Together, these synapomorphies define much of what makes the functional biology of echinoderms distinctive from that of other metazoans.
-
Calcitic skeleton composed of many ossicles.
The biomineral matrix of echinoderm skeletons is composed of calcium carbonate and several proteins. The calcite is deposited as numerous tiny crystals, but all of them lie on the same crystal axis within an ossicle. For this reason, ossicles are birefringent under polarizing light. Ossicles are not solid, but have a sponge-like microstructure called stereom that is unique to the phylum. Embryologically, echinoderm ossicles are a true endoskeleton, since they are produced by mesenchymal cells and are usually covered by epidermis. Functionally, however, the majority of ossicles act more like an exoskeleton, lying just under the epidermis and enclosing most other tissues in a flexible but tough covering.
-
Water vascular system.
The water vascular system performs many important functions in echinoderms, including locomotion, respiration, and feeding; in addition, most sensory neurons are located at the termini of podia (tubefeet) which are part of this organ system. The water vascular system may have evolved from simple tentacular systems similar to those in other deuterostome phyla, such as the tentacles of pterobranch hemichordates. However, there are many derived features of the water vascular system in echinoderms, including: an embryological origin from left mesocoel, podia arranged along branches (ambulacra), and a central circumesophageal ring.
-
Mutable collagenous tissue.
The ossicles of echinoderms are connected by ligaments composed predominantly of collagen. The material properties of this connective tissue are mutable on short timescales, under neuronal control. Ligaments are normally "locked" (rigid), but can be temporarily "unlocked" (loosened). This provides some interesting mechanical advantages, including the ability to maintain a variety of postures with no muscular effort. In holothuroids, which contain only microscopic ossicles, the entire body wall contains mutable collagenous tissue.
-
Pentaradial body organization in adults.
The adults of all extant echinoderms are radially symmetrical. A superficial bilateral organization has evolved twice, in irregular echinoids and holothuroids, but is based on an underlying five-fold organization of skeleton and most organ systems, and is clearly secondary. Higher order radial symmetry (e.g., seven-fold or nine-fold) has evolved on several occasions, and is also clearly a secondary modification. The evolutionary origins of five-fold symmetry remain obscure. Some early Paleozoic echinoderms are not radially symmetrical (e.g., carpoids and helicoplacoids), while a possible echinoderm from the Vendian (Arkarua) has five-fold radial body organization.
Plesiomorphies and other features
-
Marine habit.
All extant echinoderms live in the ocean, and there is no fossil evidence of any exception to this. Within the marine realm, echinoderms occupy nearly all habitats, where they often constitute a major proportion of the biomass.
-
Pelago-benthic life cycle.
With rare exception, echinoderms are gonochoric (separate sexes) with no overt sexual dimorphism. Fertilization is almost always external. Ancestrally (and still, typically), the life cycle is complex, with a free-living larva that is planktotrophic (grazes on unicellular algae). Larvae are plesiomorphically bilaterally symmetrical, have a recurved gut and transparent ectoderm, and feed by upstream particle capture using the ciliated band. Metamorphosis is typically radical and occurs during settlement onto the benthos.
-
Coelomate.
Echinoderms form their coeloms as outpocketings from the archenteron (embryonic gut), a process called enterocoely. In most species, the coeloms are trimerous, and initially bilaterally symmetrical. The fates of the various coelomic compartments vary among echinoderms, but some features seem broadly similar and may reflect a common evolutionary origin deep within the phylum: left mesocoel gives rise to most or all of the water vascular system, and one or both somatocoels form the lining of the body cavity.
-
Deuterostome.
Like some related phyla, the blastopore (site where gastrulation begins) in echinoderm embryos becomes the larval anus; the larval mouth is a secondary opening. In some extant forms, the larval mouth is preserved as the adult mouth, while in others the entire digestive system is re-plumbed during metamorphosis and a new mouth and anus form.
-
Simple hemal/excretory system.
The hemal and excretory systems of echinoderms are linked into what Nielsen (1996) calls the "axial complex". This organ system shows similarities, and may be homologous, to those of other deuterostome phyla. In echinoderms, it is composed of: a thickened vessel (the "heart") lacking an endothelium and surrounded by a pericardium; a region where ultrafiltration occurs via podocytes; a closed circulatory system; and an opening to the external environment called the madreporite.
-
Decentralized nervous system.
The arrangment of the central nervous sytem of echinoderms is quite different from that in other deuterostomes. Radial nerves run under each of the ambulacra, and contain the cell bodies of almost all motor neurons and interneurons. A central nerve ring surrounds the gut, and is composed primarily of fiber tracks connecting the radial nerves. No known echinoderm contains anything that could be called a brain, although ganglia are present along the radial nerves in some echinoderms. Unlike most bilaterian phyla, echinoderms lack any trace of cephalization, and have no specialized sense organs. Sensory neurons are located primarily within the ectoderm of podia, and send axons to the radial nerves.
Discussion of Phylogenetic Relationships
With the possible exception of two species, all extant echinoderms fall into five well-defined clades, traditionally ranked as Classes: the Crinoidea, Asteroidea, Ophiuroidea, Echinoidea, and Holothuroidea. The monophyly of these Classes is not in serious doubt. The phylogenetic position of two species, however, remains unresolved at the Class level. Both species belong to the recently-described "sixth" class, the Concentricycloidea; they may be highly derived asteroids, perhaps related to caymanostellids (Baker et al. 1986; Smith 1988b), but this remains controversial (Pearse 1994; Mooi et al. 1997).
Phylogenetic relationships among the five extant classes have been the subject of considerable debate. Renewed interest in resolving this problem during the past decade has greatly narrowed the number of likely topologies (Littlewood et al. 1997). It is generally agreed that the lineage leading to the Crinoidea branched most basally, and that the Echinoidea and Holothuroidea are extant sister clades. Debate now centers on whether the Ophiuroidea and Asteroidea form a clade. The two best-supported hypotheses are:

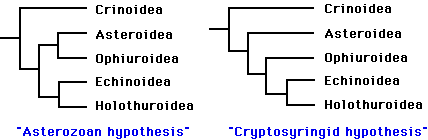
The "Asterozoan" hypothesis was first proposed by Bather (1900), and has recently been supported by the work of Mooi and David (1997). The alternative hypothesis dates back to MacBride (1906). More recently, (Smith 1988a) advocated the same topology, and proposed the name Cryptosyringida for the clade (Ophuroidea + Echinoidea + Holothuroidea). These two hypotheses are preferred, to a nearly equal degree, by molecular analyses (18S and 26S rRNA sequences) and "total evidence" analyses (summarized in Littlewood et al. 1997). Mitochondrial gene order seems to support the Asterozoan hypothesis (Smith et al. 1993), but information from crinoids is needed to clarify the polarity of the character transformations.
Including fossil taxa, the number of echinoderm classes rises to approximately 20. The lack of a clear understanding of homologies among ossicles in various extinct classes has hampered attempts to reconstruct their phylogenetic relationships (Mooi and David 1997), and it is fair to say that much work remains to be done. Analyses by Smith and Paul (1984), Smith (1988a), and Sumrall and Sprinkle (1997) are the most comprehensive and rigorous available for Paleozoic echinoderms.
References
Bather, F.A. 1900. The Echinodermata. Part iii, A and C of A Treatise on Zoology (R.R. Lankester, ed.). Black, London.
Brusca, R.C., and G.J. Brusca. 1990. Invertebrates. Sinauer, Sunderland MA.
David, B. and R. Mooi. 1997. Major events in the evolution of echinoderms viewed by the light of embryology. In Echinoderms: San Francisco (R. Mooi and M. Telford, eds.). Balkema, Rotterdam. In press.
Gehling, J.G. 1987. Earliest known echinoderm -- a new Ediacaran fossil from the Pound Subgroup of South Australia. Alcheringa 11: 337-345.
Hyman, L.H. 1955. The Invertebrates, vol. iv, Echinodermata. McGraw-Hill, New York.
Littlewood, D.T.J. 1995. Echinoderm class relationships revisited. Pages 19-28 in Echinoderm Research 1995 (R. H. Emson, A. B. Smith, and A. C. Campbell, eds.). Balkema, Rotterdam.
Littlewood, D.T.J., A. B. Smith, K. A. Clough, and R. H. Emson. 1997. The interrelationships of the echinoderm classes: morphological and molecular evidence. Biol. J. Linn. Soc. 61: 409-438.
MacBride, E.W. 1906. Echinodermata. In The Cambridge Natural History (S.F. Harmer and A.E. Shipley, eds.). MacMillan, London.
Mooi, R., F.W.E. Rowe, and B. David. 1997. Application of a theory of axial and extraxial skeletal homologies to concentricycloid morphology. In Echinoderms: San Francisco (R. Mooi and M. Telford, eds.). Balkema, Rotterdam. In press.
Mooi, R., and B. David. 1997. Skeletal homologies of echinoderms. Pal. Soc. Papers, in press.
Nielsen, C. 1995. Animal Evolution: Interrelationships of the Living Phyla. Oxford University Press, Oxford.
Paul, C.R.C. and A.B. Smith. 1984. The early radiation and phylogeny of echinoderms. Biol. Rev. 59: 443-481.
Pearse, V.B., and J.S. Pearse. 1994. Echinoderm phylogeny and the place of the concentricyloids. Pages 121-126 in Echinoderms through Time (B. David, A. Guille, J.-P. Féral, and M. Roux, eds.). Balkema, Rotterdam.
Raff, R.A., K.G. Field, M.T. Ghiselin, D.J. Lane, G.J. Olsen, N.R. Pace, A.L. Parks, B.P. Parr, and E.C. Raff. 1988. Molecular analysis of distant phylogenetic relationships in echinoderms. Pages 29-41 in Echinoderm Phylogeny and Evolutionary Biology (C.R.C. Paul and A.B. Smith, eds.). Clarendon Press, Oxford.
Rowe, F.W.E., A.N.Baker, and H.E.S. Clark. 1988. The morphology, development and taxonomic status of Xyloplax Baker, Rowe and Clark 1986 (Echinodermata:Concentricycloidea), with the description of a new species. Phil. Trans. R. Soc. Lond. B 233: 431-459.
Smith, A.B. 1984. Classification of the Echinodermata. Paleontology 27:431-459.
Smith, A.B. 1988a. Fossil evidence for the relationship of extant echinoderm classes and their times of divergence. Pages 85-97 in Echinoderm Phylogeny and Evolutionary Biology (C.R.C. Paul and A.B. Smith, eds.). Clarendon Press, Oxford.
Smith, A.B. 1988b. To group or not to group: taxonomic position of Xyloplax. Pages 17-23 in Proceedings of the 6th International Echinoderm Conference (R. D. Burke, P. V. Mladenov, P. Lambert, R. L. Parsley, eds.). Balkema, Rotterdam.
Smith, M.J., A. Arndt, S. Gorski, and E. Fajber. 1993. The phylogeny of echinoderm classes based on mitochondrial gene rearrangements. J. Mol. Evol. 36:545-554.
Sumrall, C. and J. Sprinkle. 1997. Phylogenetic analysis of living Echinodermata based on primitive fossil taxa. In Echinoderms: San Francisco (R. Mooi and M. Telford, eds.). Balkema, Rotterdam. In press.
Wada, H., and N. Satoh. 1994. Phylogenetic relationships among extant classes of echinoderms, as inferred from sequences of 18S RNA, coincide with relationships deduced from the fossil record. J. Mol. Evol. 38:41-49.
Information on the Internet
- The CAS Echinoderm Webpage. California Academy of Sciences.
- Introduction to the Echinodermata. UCMP Berkeley.
- Phylum Echinodermata. University of Michigan Museum of Zoology Animal Diversity Web.
- Echinodermata. BIOSIS BiologyBrowser.
- Echinoderms of Hawaii. Hawaii Coral Reef Network.
- Underwater Field Guide to Ross Island & McMurdo Sound, Antarctica: Seastars and Urchins, brittle stars, sea cucumbers, crinoids.
- Bathyal and Abyssal Echinoderms. Deep-Sea Pages by Paul H. Yancey, Whitman College.
- Gallery of Echinoderm Images. Swedish Museum of Natural History.
- Cladestrat. A data base at the University of Bristol containing results of tests to compare cladograms with stratigraphy. Several echinoderm data sets are included.
- The Echinoid Directory. Andrew B. Smith, The Natural History Museum, London.
- Sea Urchin Embryology. A series of laboratory modules developed by high school teachers and Stanford University researchers.
- Growth of a Starfish. Micscape Magazine.
Title Illustrations

| Scientific Name | Crinoidea |
|---|---|
| Location | Palau, Micronesia |
| Comments | Feather star |
| Specimen Condition | Live Specimen |
| Copyright | © 1998 Edoardo Bellotti, e&c Photo Gallery |
| Scientific Name | Echinaster sepositus |
|---|---|
| Specimen Condition | Live Specimen |
| Identified By | id confirmed by Christopher Mah (from photograph) |
| Copyright |
© Peter Wirtz

|
| Scientific Name | Ophioderma rubicundum |
|---|---|
| Location | Grand Cayman, British West Indies |
| Comments | Ruby brittle star on brain coral |
| Specimen Condition | Live Specimen |
| Copyright | © 1998 M. Benjamin Cowan, Ocean Images |
| Scientific Name | Strongylocentrotus purpuratus |
|---|---|
| Location | Cape Perpetua, Oregon, USA |
| Comments | Sea urchin |
| Specimen Condition | Live Specimen |
| Image Use |
 This media file is licensed under the Creative Commons Attribution-NonCommercial License - Version 2.5. This media file is licensed under the Creative Commons Attribution-NonCommercial License - Version 2.5.
|
| Copyright |
© 1997
Katja Schulz

|
About This Page
Many thanks to Rich Mooi, Olaf Ellers, and Emily Knott for help in assembling this page.
Gregory A. Wray

Duke University, Durham, NC, USA
Correspondence regarding this page should be directed to Gregory A. Wray at
gwray@duke.edu
Page copyright © 1999 Gregory A. Wray
All Rights Reserved.
- First online 14 December 1999
Citing this page:
Wray, Gregory A. 1999. Echinodermata. Spiny-skinned animals: sea urchins, starfish, and their allies. Version 14 December 1999 (under construction). http://tolweb.org/Echinodermata/2497/1999.12.14 in The Tree of Life Web Project, http://tolweb.org/












 Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site
Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site